Newly discovered sleep biomarkers pave the way for early detection of Parkinson’s disease, Alzheimer’s disease, Lewy body dementia, and other neurodegenerative diseases. They may also one day lead to interventions.
By Risa Kerslake, BSN-RN
Many people with neurodegenerative diseases have altered sleep due to neurodegenerative changes brought on by damage or loss of neurons in the sleep-controlling centers of the brain or as a result of disease symptoms.1 For example, Parkinson’s disease-related movement difficulties make it challenging to get comfortable in bed, while Alzheimer’s disease-linked long daytime naps often lead to insomnia.
As growing evidence links neurodegenerative diseases and sleep disturbances, managing underlying sleep disorders may improve neurodegenerative effects,2 increasing the overall quality of life.
Discoveries of novel sleep biomarkers can facilitate a better understanding of overall health and serve as tools to identify both sleep and neurodegenerative diseases, paving the way for the differential diagnosis of disorders such as dementia with Lewy bodies and earlier detection of neuronal cell loss in patients.
RBD as a Precursor to Neurodegenerative Diseases
More than 70% of adults with REM sleep behavior disorder (RBD) will eventually develop neurodegenerative diseases such as Parkinson’s disease or Lewy body dementia.3
“Since the early 2000s when it began to become obvious that REM sleep behavior disorder was the beginning of a neurodegenerative disease in many older adults who develop it, physicians worldwide grew interested in studying it cooperatively,” says Erik K. St. Louis, MD, MS, associate professor of neurology and co-director of the Center for Sleep Medicine at Mayo Clinic.
As a result, the International RBD Study Group was formed and has published the largest natural history studies of REM sleep behavior disorder (RBD) to date.4
More recently, the North American Prodromal Synucleinopathy (NAPS) Consortium brought together nine academic medical centers to study RBD to inform neuroprotective treatment trials. “Several promising-appearing medications are being developed that one day may be offered in a neuroprotective clinical trial to halt or slow the progression of neurodegeneration in patients with isolated REM sleep behavior disorder that could prevent the development of Parkinson’s disease or dementia with Lewy bodies,” says St. Louis, who is also co-director of the NAPS consortium.
St. Louis has been collaborating with sleep diagnostics and therapy company Advanced Brain Monitoring to produce a home study approach using devices that incorporate electroencephalogram (EEG) and electromyography (EMG) channels to diagnose RBD in community settings. “I am increasingly excited about efforts to diagnose REM sleep behavior disorder based on a simpler home study approach, using devices as simple as wrist actigraphy or other home sleep diagnostic studies that incorporate EEG and EMG channels to accurately diagnose REM sleep behavior disorder in patients in broader communities without the need for laboratory-based studies,” St. Louis says.
Discovering Sleep Biomarkers of Neurodegeneration
When looking at neurodegenerative diseases, clinicians hope to identify unique characteristics of sleep that can differentiate neurodegenerative disease types.
Daniel Levendowski, founder of Advanced Brain Monitoring, and co-investigators have so far identified two novel sleep biomarkers that could be used for this purpose: non-REM sleep with hypertonia5 and sleep spindle duration.6
The discovery of non-REM sleep with hypertonia was serendipitous. Its signal emits only from the brain’s frontal region—so it has never been detectable by the standard polysomnography montage (in which the electrodes are placed on the hairline). Advanced Brain Monitoring’s home sleep test, marketed as Sleep Profiler, uses forehead electrode placement, which it only did for patient ease of use. “We wanted to have the patient put the device on themselves. You can’t put electrodes on top of hair easily at home,” Levendowski says.
When zooming out to 30-minute periods to better see REM periods in Sleep Profiler’s software, Levendowski saw a signal in patients with dementias that first elevated, then stayed high for a time, and finally came back down. “When we did simultaneous recordings with Sleep Profiler and polysomnography in people who were suspected of having RBD, there was nothing about what occurred in that video that indicated any activity that could explain it,” Levendowksi says.
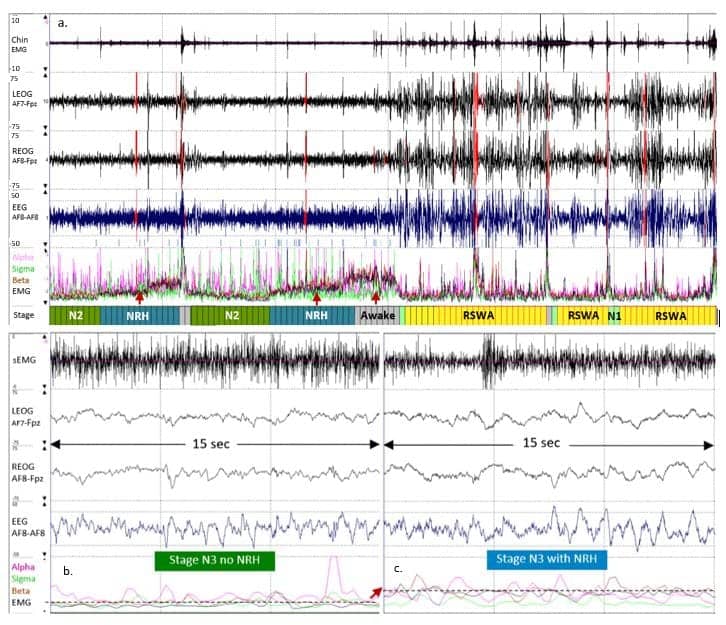
The potential utility of non-REM sleep with hypertonia as a sleep biomarker only became apparent during a review of the data set, which included patient populations with prodromal or manifest Parkinsonism. If the Sleep Profiler data shows non-REM sleep with hypertonia, “then you have something that can begin to separate one group from another group,” says Levendowski.
Sleep spindles, bursts of brain activity that occur during non-REM sleep, are known to be linked to cognition, especially to learning and memory. But Levendowski and co-investigators look at spindles a little differently than other researchers. “It’s not how many spindles a person has per hour, but how much spindle time a person gets over the course of the night. That’s your exposure to helping memory consolidation,” Levendowski says of the novel neurodegeneration biomarker of spindle duration. “I’ve seen cases of people with Lewy body dementia in which they have normal-looking density [the traditional sleep spindle metric] but very abnormal duration.”
In most people, sleep spindle density and sleep spindle duration are equivalent. “Most studies are on memory in healthy people; duration versus density doesn’t matter in healthy people,” he says. But reduced sleep spindle duration may be a distinct sleep biomarker for neurodegenerative disorders, likely indicating thalamocortical dysfunction.
Correlations are “very poor” between sleep spindles and non-REM sleep with hypertonia across all the different neurodegenerative disease groups, Levendowski adds. “This means every new marker that’s independent of the other one is adding unique value that can contribute to the differential diagnosis,” he says.
Next, the investigators will look at Alzheimer’s disease and Lewy body dementia over time to determine whether patterns are stable or fluctuating, analyzing the degree of stability from night to night.
Leveraging Machine Learning for Analysis
The sleep disturbances that signal underlying neurodegenerative diseases can be subtle, making them difficult to extract reliably over long recordings using traditional methods, explains Jacob Donoghue, MD, PhD, CEO of Beacon Biosignals, an EEG neurobiomarker platform. By analyzing hundreds of thousands of hours of data across a database of diverse patient populations, machine-learning tools at Beacon can identify and extract biomarkers associated with neurodegenerative diseases.
Machine learning allows computer systems to learn from data, identify patterns, and make decisions with minimal human intervention. “Our approach involves training machine-learning algorithms using thousands of assessments made by multiple expert sleep clinicians,” says Donoghue. These experts assess PSG data to identify events such as epileptiform activity or sleep macro- and micro-architecture patterns. Algorithms learn from this input and enable the identification of similar events in new data.
Beacon Biosignals recently enhanced the reach of its analytics platform with the acquisition of Dreem, an at-home sleep monitoring company that produces a sleep headband-based dry electrode EEG system. Prior to the acquisition, Dreem had recorded over 2 million nights of sleep in at-home users. By integrating Dreem’s technology with Beacon Biosignals’ machine-learning platform, Donoghue says his team unlocked the potential for artificial intelligence-enhanced EEG analysis at scale.
“This not only furthers our potential for understanding the etiology of sleep pathology in neurodegenerative diseases, such as studying the role RBD plays in the progression of Parkinson’s, but also enables us to contribute to the development of targeted therapies for these disorders,” Donoghue says, noting that all of Beacon’s work is with biopharma companies supporting the development of such treatments.
Nervous System Clues to Neurodegenerative Diseases
SleepImage markets a US Food and Drug Administration-approved Software as a Medical Device to measure sleep via the autonomic nervous system. The SleepImage Ring and fingertip devices can detect fluctuations of cardiovascular and pulmonary function during sleep using technology based on two fundamental signals: heart rate variability and breathing.
“People with a genetic predisposition for some neurodegenerative diseases often experience autonomic symptoms prior to cognitive or motor issues,” says Jeff Durmer, MD, PhD, chief medical applications officer with SleepImage.
SleepImage’s cardiopulmonary coupling algorithm uses a mathematical formula that includes the coherence of heart rate variability and tidal volume fluctuations calculated with cross-spectral power to see how coherent, or coupled, these signals are. “The more coupled the signals are, the more vagal activity or parasympathetic tone is noted. When you see deep stages of non-REM sleep, that’s when parasympathetic vagal tone is increased and where cardiopulmonary coupling is elevated,” Durmer says.
In the last 20 years, neurologists have started to appreciate the concept of autonomic dysfunction appearing in association with neurodegeneration. Autonomic dysfunctions that cause sleep problems can provide clinicians with an opportunity to implement therapies that improve autonomic nervous system rest and recovery. This might involve adjusting medications that activate the sympathetic nervous system, such as amphetamine-based stimulants, or addressing medical conditions like uncontrolled diabetes that stress the autonomic nervous system. Improving sleep quality can have a significant impact as a modifiable risk factor for neurodegeneration, Durmer explains.
One of the most exciting things to Durmer is using SleepImage’s software to see a person’s sleep “fingerprint.” The individualized sleep data enables clinicians to tailor patient recommendations based on quantitative metrics.
“We’re in an era of personalized medicine,” says Durmer, “You wear a ring on a finger, we analyze your pulse using plethysmography throughout the night, and we’ve got the ability to help you. This is a great public health opportunity to impact large populations by utilizing the window of sleep to improve health care.”
References
- Malhotra RK. Neurodegenerative disorders and sleep. Sleep Med Clin. 2018 Mar;13(1):63-70.
- Shen Y, Lv QK, Xie WY, et al. Circadian disruption and sleep disorders in neurodegeneration. Transl Neurodegener. 2023 Feb 13;12(1):8.
- Roguski A, Rayment D, Whone AL, et al. A neurologist’s guide to REM sleep behavior disorder. Front Neurol. 2020 Jul 8;11:610.
- Joza S, Hu MT, Jung KY, et al; International REM Sleep Behavior Disorder Study Group. Progression of clinical markers in prodromal Parkinson’s disease and dementia with Lewy bodies: a multicentre study. Brain. 2023 Aug 1;146(8):3258-72.
- Levendowski DJ, Walsh CM, Boeve BF, et al. Non-REM sleep with hypertonia in Parkinsonian Spectrum Disorders: A pilot investigation. Sleep Med. 2022 Dec;100:501-10.
- Levendowski D, Walsh C, Boeve B, et al. 0266 Sleep spindle-duration: A potential biomarker for neurodegenerative disorder phenotyping. Sleep. 2022 June;45(issue suppl_1):A120.
Illustration 20980453 © Eugenp | Dreamstime.com