As the first FDA-cleared, fully implanted neurostimulation device for obstructive sleep apnea becomes available in additional cities throughout the United States, Sleep Review provides guidance on how to screen patients for this therapy.
By C.A. Wolski
Inspire Medical System Inc’s implantable device for the treatment of obstructive sleep apnea (OSA) is the first treatment of its kind cleared by the Food and Drug Administration (FDA). While it is designed for patients who can’t be treated with traditional clinical methods, it isn’t a panacea.
In fact, both the FDA and Inspire Medical have developed guidelines designed to help clinicians make sure the patient fits the medical criteria that are needed for Inspire Upper Airway Stimulation to be an appropriate therapy. While the criteria resemble the screening patients will undergo for the traditional treatments, Inspire screening is more comprehensive—evaluating everything from sleep hygiene to patient anatomy.
Developing the Criteria
Going hand-in-glove with the development of Inspire, which requires an outpatient procedure by an otolaryngologist, the company, with guidance from the FDA, also developed criteria that clinicians need to follow to find the most suitable patients for the therapy. Because the therapy is invasive, it can’t be a first line treatment.
“The patient has to have documentation that they have tried CPAP for at least 3 months,” says Quan Ni, PhD, VP of research at Inspire Medical. Ni led the team that developed the criteria following the clinical trials.
The patients in the original study group had, on average, been diagnosed with OSA at least 5 years prior to undergoing Inspire therapy and had an average age of 55.
In addition to having been unsuccessfully treated with CPAP—which has been documented by a treating physician—potential patients must have an apnea-hypopnea index (AHI) of between 20 and 65. The FDA has set no contraindication for obesity, but Ni says that Inspire recommends that patients have a BMI below 32.
Candidates for Inspire also must undergo a sedated endoscopic procedure during the evaluation process, allowing an ENT physician to observe the patient’s anatomy in a sleep-like state. “We’re looking for a very specific anatomy during the procedure,” Ni says. “We’re looking for a complete concentric collapse at the level of the soft palate. If the patient has a lateral wall collapse in addition to anteroposterior collapse of the soft palate, that’s a contraindication for Inspire.” Ni adds that about 10% to 20% of Inspire candidates cannot get the device due to this contraindication.
Inspire in Action
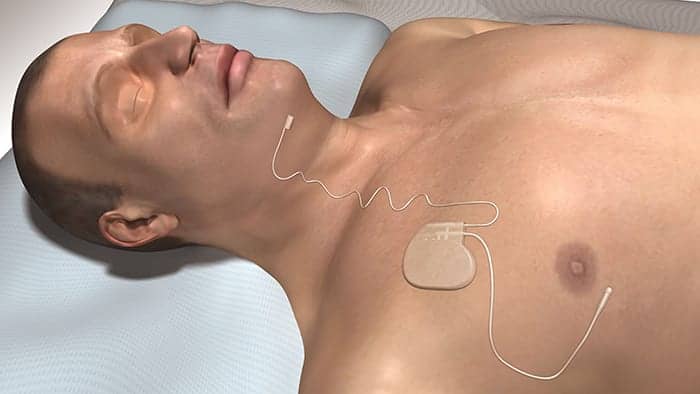
[caption id="attachment_135404" align="aligncenter" width="300"] Patients activate Inspire therapy with a remote that gives them about 30 minutes to fall asleep.[/caption]
Patients activate Inspire therapy with a remote that gives them about 30 minutes to fall asleep.[/caption]
Since it was approved for use by the FDA in 2014, hundreds of patients have benefited from the therapy. Ryan Soose, MD, an ENT and director of the University of Pittsburgh Medical Center (UPMC) Division of Sleep Surgery, has implanted about 50 of the devices with good results.
Prior to implanting the device, Soose determines that the patient’s CPAP or dental device is, in fact, not helping to treat OSA. “CPAP, the standard first line therapy, is a robust treatment, but about 50% to 60% of patients don’t use or can’t use it. We troubleshoot the CPAP [device] and see if the patient can actually use it effectively,” he says.
Soose says that patients who have used dental devices to treat their OSA may have changes in their teeth or bite patterns because of these devices that then make the dental devices difficult or ineffective to use.
Once he determines the traditional therapies are, indeed, not working for the patient, he has the patient undergo a traditional sleep study, evaluates the patient’s body weight, then the anatomical phenotype through the use of a sedated endoscopy, and reviews the quality of the data and scientific literature (Ni says Inspire Medical is in the process of conducting ongoing scientific studies of the implantable therapy in the United States and Germany). Soose then weighs the pros and cons of treatment for the patient. “The patient that’s best has moderate to severe OSA, is not significantly overweight, and has specific, favorable anatomy,” he says.
Another factor that has to be weighed by the clinician when evaluating the patient is that the current Inspire model is not MRI compatible. If a patient needs to undergo an MRI procedure, the device must be removed.
Because the device requires the patient to turn it on at night, they have to have some manual dexterity in order to operate the device’s remote.
The surgery itself is a relatively simple outpatient procedure, and involves no cutting or rearranging of the throat or jaw.
Soose’s patient referrals come through many of the sources that would be expected—primary care physicians, cardiologists, and sleep physicians—but a significant number of patients are self-referred, finding physicians who are trained to implant the Inspire therapy device through the company’s website. (The physician finder tool is simple. Potential patients can simply input their ZIP code, and the names and contact details of physicians nearest them will be delivered in a matter of seconds.)
The Story of Denise H.
Denise Hoover (name used with patient’s permission) discovered the existence of Inspire therapy the old-fashioned way—she read an article in the newspaper. And it came at a fortuitous time.
Diagnosed with OSA about 20 years ago, she underwent surgery to remove her tonsils and uvula. “That worked for awhile, then I used CPAP and I couldn’t tolerate that, then I tried a dental appliance and that didn’t work. I was out of options,” she says.
Hoover first consulted with Patrick Strollo, MD, FCCP, FAASM, medical director of the Sleep Medicine Center at UPMC, about having the Inspire device implanted.
Strollo ordered a sleep study, which Hoover knew would show that she had OSA. She then followed up with Soose, who evaluated her CPAP tolerance and checked to make sure she fit the BMI criteria. Finally, she underwent a sedated endoscopy that showed she was a good candidate for the procedure, which she underwent in May 2014.
Soose notes that many of his Inspire patients know they are difficult cases. “These aren’t patients that are expecting to eliminate their OSA. They’re more accepting of the side effects of OSA treatment,” he says. There are some potential side effects from the treatment, according to the company’s website, ranging from dry mouth or trouble speaking to nerve damage or bleeding.
As with CPAP and other traditional OSA treatments, patients are required to take part in activating the Inspire device nightly. Patients turn on the device with a remote that gives them about 30 minutes to fall asleep. This allows the treatment to be administered very gradually, not interfering with patients’ natural sleep cycle, Ni notes. “As the patient falls asleep, the treatment isn’t very noticeable,” he says.
During follow-up exams, patients are held accountable. A transponder in the device allows clinicians to measure the frequency with which patients use the device. The early version of the device did not allow clinicians to measure the number of hours that patients used it, Ni notes, but self-reporting surveys in the clinical trials had 80% of patients self-reporting that they were using it regularly.
Overcoming Failure
Not every patient who wants Inspire therapy will be able to receive it due to a single or combination of factors—AHI, anatomy, or BMI.
Soose doesn’t see the Inspire therapy as a new magic bullet that will “cure” OSA in those difficult cases. Instead he’s part of a new mindset that sees combination therapy as the route to success. “Patients aren’t well served with a cookie-cutter approach that starts with CPAP and ends with aggressive surgeries,” Soose says. “Today we’re doing more customized therapies, such as weight loss or a combination of therapies. Inspire filled a nice void. It’s shown to be successful with the right clinical and anatomical criteria. You don’t have to hit a home run with therapy.”
Soose, who helped develop the Inspire training program, notes that there are three criteria for a successful outcome: proper patient selection, correct execution of the implant, and proper follow-up (including proper therapy titration). The Inspire training program outlines all of these aspects in detail. “Our goal is to build a comprehensive program,” Ni says.
Currently, there are 30 centers that offer Inspire therapy, which can be found through the website.
Watch a 30-minute video about Inspire therapy.
C.A. Wolski has seen OSA technology come a long way since he first started reporting on sleep medicine in 2001. He’s particularly inspired by the combination therapy idea being put in practice by Ryan Soose, MD, and others.






This was a great alternative to the CPAP machine for me and really has changed my life.
Are you still enjoying your Inspire? What symptoms did you present to get insurance to approve?
Will inspire work for apnea due to night time congestion
I stop breathing because I plug up & gasp
My cpap machine x8 years has yet to help me
Must yoU try the CPAP before you can try Inspire? My husband was diagnosed with moderate Sleep Apnea over 15 years ago in a sleep study . He refused to get the CPAP machine. His snoring is very loud, and he must sleep in another room. We are interested in “Inspire”. Must he have tried CPAP first?. He would prefer to try the Inspire. Thx!
I am iinteresting !
This is wonderful news for those of us who HATE and have HOPED for years and years that we might, we JUST MIGHT, be able to get rid of the annoying mask one of these days. This makes that hope brighter and brighter
Does inspire work for dot truck drivers that require CPAP machine down loads every six month to show your using it at least four hours a night or by new government law can’t drive or work
I’ve have CPAP did sleep study and put me under for video of inside breath air to see if good candidate and was told yes but but is far as I am so far haven’t met with docters that does implant
I can’t say for sure it’s accepted by the DOT, but the Dr. can print out a report showing you are using Inspire therapy each night, so I would think that would be like your CPAP downloads. And it’s possible the DOT would require a follow up sleep study to prove it’s working.
Looking forward to evaluation. I meet some of the criteria. CPAP is not working; mandibular appliance was evaluated: bad jaw and tooth structure. Now there might be hope.
I am very interested, dislike my cpap machine it wakes me 3 times a night and do not get a good nights rest. I struggle with it nightly. I ware it because I have to. although not getting the proper rest leaves me tired and anxious. I Would like to have a consult
Hi Laura, To find a doctor who offers Inspire therapy, please visit InspireSleep.com/doctor-search/.
I’ve read that although DOT requires truckers to use the cpap device if diagnosed, the data on crash studies shows, having sleep apnea did not contribute to the accidents. I wonder why this law was still implemented. Ironically, the one thing I see as a problem, is wearing the darn thing, which I have, and lost sleep using it. If I was scheduled to drive, lack of sleep could put myself and others at risk.
I would assume the inspire device would be more acceptable for DOT medical card, if it results in getting better sleep. I’m seriously thinking about the Inspire implant. The side effects, and warnings are a bit of concern.
Do you know if Káiser Permanente cobres the Ispire Device? Te
I don’t think Kaiser offers it in San Francisco, perhaps in other areas. The VA in SF does offer it. UCSF does have one doc who is doing the procedure. Peter
Kaiser in Northern California offers Inspire. I am in the process of inquiring about it. Have had CPAP tests but cannot get used to the mask. I will contact Kaiser tomorrow to find out about it. Kaiser does offer it though.
I’m interested. I can’t use the machine, my nose plugs up since I have a narrow air passage.
I would very interested in this study. I have had sleep apnea for many years now and can not keep the mask on during the night. I stopped using the machine because of this reason, any alternative would be great.
I was first diagnosed in 2011 with OSA and an AHI of 92/hour! I adjusted to CPAP with no problems; I’m on 10 cm with oxygen at 3L. In 2016 I was diagnosed with complex sleep apnea. I don’t have my results, so I can’t tell you my AHI. I’m waiting to be started with BiPap at 21/18. I am totally exhausted with terrible morning headaches. Can Inspire possibly help me? I have a BMI of 26. Thank you!
Hi Roseann, Inspire therapy can help with complex sleep apnea, depending on the ratio of obstructive apneas to central apneas. If less than 25% of your total events stem from central sleep apnea, you may be a candidate for Inspire therapy.
I am so interested!!!!!
Still wake up on my cpac machine with 2 liter two to three times does insurance cover it
Hi Calvin, Inspire therapy is being reviewed and approved on a case by case basis. Your Inspire physician will work on your behalf to gain approval.
My Bypap doesn’t work the dental products don’t work. I never dream cause I can’t get to level 5 sleep. I’m tired all the time, and I have no energy and I’m only 54. Would love to try this trial device I want to sleep for more than 2 hrs at a time!
I need this so. I can’t handle the CPAP machine. The mask never stays on. I’m so glad that my insurance will pay for this wonderful implant. I need it so. I’m always sleepy and want to sleep all day if I could. I am retired and would feel good again.
I was diagnosed with severe apnea for 10 years not comfortable with cpap machine coz it aggravate my sinusitis that resulted in surgery, waisted my money with dental implant. Had trouble memorizing and remembering information, failed many time in my licensing exam, embarrassed due to marks in my face in wearing mask, I’ll be glad to considered as a candidate for implant. I hope Kaiser Permanente Insurance will cover it.
Does medicate and tricare cover this device. I’ve been on clap for 2 or 3 years but my wife says that I keep pulling it off at night I can’t find a mask that I can handle. My pulmonoligist wanted to put me on bipap but the company I’m with hasn’t responded yet that was Sept. 2015. I’m also on oxygen 25/7. Please help. I had to give up my license because of not sleeping at night so I had a tendency to fall asleep at the wheel.
Hi Richard, Inspire therapy is being reviewed and approved by Medicare and Tricare on a case by case basis. Your Inspire physician will work on your behalf to gain approval for the device. Please visit inspiresleep.com to learn more about Inspire therapy.
I am using the ACAP for two years it is fine I have not complain bud if this new devise is better I will like to see if I am a candidate, I have Medicare A/B as also Blue Cross Blue Shield the cover the cost?
Hi Martin, Inspire therapy is being reviewed and approved by insurance providers on a case by case basis. Your Inspire physician will work on your behalf to gain approval.
as I understand there is two types of sleep apnea that is considered with inspire
nbr 1 central sleep apnea
nbr 2 obstructive sleep apnea
I am told my sleep apnea is central which is no acceptable to inspire is that correct ?
thank you in advance of your response
Bill
Hi Bill, You are correct–Inspire therapy is only for obstructive sleep apnea or mixed sleep apnea (a mix of obstructive and central) as long as less than 25% of your apnea events are from central sleep apnea.
I had severe central apneas at every CPAP pressure so I am using an ASV machine. It appears I am not a candidate for Inspire since I only have central apneas. Is that correct? Thank you!
Hi Connie, You are correct–Inspire therapy is only for obstructive sleep apnea or mixed sleep apnea (a mix of obstructive and central) as long as less than 25% of your apnea events are from central sleep apnea.
I been on cpap for few years,works good for a while but now I just ca’t get a fresh morning,wake up tired and with headache most of the time,I have health net insurance true work,do you take this insurance?..,I’m 45 years young
Hi Eric, Inspire therapy is being reviewed and approved by insurance providers on a case by case basis. You will need to call the clinic directly to see what type of insurance they take. You can see a full list of doctors who offer Inspire therapy by visiting InspireSleep.com/doctor-search/.
I have obstructive sleep apnea for 7-8 years as well as chronic insomnia . Been through numerous sleep studies that verified my condition .No luck w/ CPAP AT 17psi various masks etc, I’m a big frame large head,frame, throat circumference etc at 6’1″ 315lbs. Problem doc said w/ minimal REM & Delta sleep losing weight is ALMOST impossible so it’s a cyclical condition . Any thoughts if Inspire has anything to offer me?
Hi Rich, Unfortunately you would not be a candidate for Inspire therapy at this time, as your BMI is too high (Inspire therapy is indicated for BMI<32). For a BMI of 32 standing at 6'1, you would need to weigh approx. 245 lbs. The BMI indication is in place due to the results of clinical findings during the Inspire therapy trials.
I have extremely severe OSA. Been told that there is no surgery that can reduce it alot or cure the apnea. I have a BMI of 38. I have 90-95 apnea’s per hour.Any suggestions or do i wait to die.
Where are the locations of the centers where the testing is done for potential
candidates?
Hi Shauna, You can see a full list of physicians who offer Inspire therapy by visiting https://www.inspiresleep.com/doctor-search/. To find one near you, just type in your zip code.
Any chance that this new treatment will become available for those with a BMI>32?
Does this stop snoring?
Hi Barbie,
In our clinical trial, 86% of bed partners reported soft or no snoring from their bed partner using Inspire therapy after 12 months.
I have used a CPAP (as a dust collector ), for over 5 years. I work 14 hour days/nights and the time I get for sleep is important. I’ve tried numerous mask types but they end up tossed on the floor. I’m very interested in the Inspire treatment. My question is once its inserted, can it be removed if its not helping? Also can it clear a metal detector, since my work requires me to enter one.
Hi Bill,
In the unlikely case Inspire therapy does not work for you, it can be removed. An alternative imaging system other than a metal detector should be used as to not damage the device. This is very common practice, as the same alternate scanning option needs to be used for any implanted device including pacemakers.
I currently use a CPAP for OSA, several years ago I had UPPP and several Turbinate reductions to help but they did not. I recently changed to a different ENT and he told me than they did not work because I had an enlarged tongue and the only thing that he could do would be to tie my tongue back with a cable and he said that he recommended me not to get that done. Can your technique work for people with enlarged tongues. 6′ 195 lbs. Thanks. MR
Hi Mike, Tongue size does not directly correlate with the candidacy requirements for Inspire therapy. To be a candidate for Inspire therapy, you must have moderate to severe OSA, are unable to use or get consistent benefit from CPAP therapy, are not significantly overweight, and successfully pass an airway anatomy exam. Only an Inspire therapy trained specialist can determine if the therapy is right for you–you can see a full list of available physicians by visiting https://www.inspiresleep.com/doctor-search/.
I am 45 years of age and I live in Jennings County Indiana. I have suffered from Obstructive Sleep Apnea more than 15 years. My first sleep study was in late 2000. I used a CPAP Machine for 11 years. In 2012 another sleep study was done. I switched to using a BIPAP Machine after that.
I obviously snore and stop breathing quite often. One of my jobs that I’ve done for 15 years is a school bus driver. A “normal” driver only has to have a physical every 2 years. For me, since I have OSA and use a BIPAP, I have to have one annually. This isn’t even mentioning the “suffering” that my family knows of, without the BIPAP.
I do have Anthem Blue Cross Blue Shield Insurance. Is there a chance that I could arrange to receive a “cure” for OSA?
My number is: 812-599-5133
Thank You,
Tim Gibson
Hi Tim, Blue Cross Blue Shield is reviewing and approving Inspire therapy on a case by case basis. The first step is setting up an appointment with an Inspire therapy trained doctor. Once he or she determines you are a good candidate, they will begin working on your behalf for approval. To find a doctor near you, please visit https://www.inspiresleep.com/doctor-search/ and type in your zip code.
I recently underwent the qualification procedures and became a candidate for Inspire. I look forward to the procedure and will report the results in a few weeks.
Hi Verna,
I was wondering how you have been making out with your inspire therapy….?
I am in constant a-fib controlled by Rx
Would I still be a candidate for this procedure? My c-pap setting is 11.
Hi Bruce, Only an Inspire therapy trained doctor can determine if you are a good candidate for the therapy. He or she will look at your afib device to see if there is any interaction possibility between it and Inspire. If not, then you may be a candidate.
Hello. I have been suffering from Sleep Apnea for over two years now. I have a Cpap Machine and I tried all the masks that is in out there. Nothing is helping . I feel so darn tried all the time so hard to do my job lately. I due have a appointment to talk about others options, I think this be a great one. Is there a certain weight you got to be for the inspire therapy
Hi Carolyn, Inspire therapy works best in people with a BMI of under 32. This is a calculation based on your height and weight. You can use a website like this one to find yours: http://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm
I swim a lot competitively and wonder if this would provide a problem with the implant? would swimming cause irritation or worse?
Hi Bob,
Everything is fully implanted, so you should have no irritation or other problems swimming with Inspire therapy.
I am a 48 year old veteran with OSA and PTSD. Due to anxiety I have had difficulty using a CPAP as it causes panic attacks. I have tried to get the VA to let me see a Inspire doctor to see if I qualify, but have been unable to convince my doctor as they don’t do Inspire at my location and feel only CPAP or BiPAP is the answer.
Hello, I too am a long-time sufferer of Sleep Apnea. (13yrs)
I use the CPAP however I do not feel a significant change in how I feel. I am still constantly tired.
I reside in Ontario and was wondering if this is available here.
Thank you.
Hi Terry, Unfortunately Inspire therapy is not available in Canada at this time. Please check InspireSleep.com for any major expansion news.
Is there an age restriction for the Inspire device? Will Medicare insurance cover the cost of the Inspire? Thank you.
Hi,
There is no age limit for Inspire therapy. Medicare is reimbursing the cost of Inspire therapy in various geographical regions across the United States. An Inspire doctor can tell you about their experience with Inspire/Medicare in your specific area.
I am a disabled veteran recently medically retired. My VA sleep doctor won’t even consider anything but BiPAP for me. Once I start on Tricare are their more options to see if I am a candidate?
Hi Chris,
Your options may expand, as you do not have to specifically go to a VA doctor for Inspire therapy if you have Tricare. Tricare is reviewing and approving Inspire therapy on a case by case basis. Your Inspire physician will work on your behalf to gain approval.
My husband is a trucker. He uses a CPAP that is wirelessly transmitted for usage compliance. Do you know if DOT accepts the usage of Inspire. He hates the CPAP and feels more tired and agitated than before usage.
Hi Shirlee,
Similar to CPAP, an Inspire doctor is able to print off compliance of Inspire therapy to prove sleep apnea treatment compliance to the DOT. There are truck drivers who use Inspire therapy, and they do not have any issues.
I have been an OSA sufferer for many years. Already been treated with CPAP and for the last couple of years an oxygen generator. I still don’t get a good nights sleep even with that machine. Here’s the question: I’m 62 yrs
old and I have an incomplete spinal cord injury, really not a bad one, but I do have an implanted baclofen pump for spastisity. I’m wondering what my chances would be of having this procedure done. It really looks like something that would fix my problem.
Please let me know.
Thank you
Hi Jack, During an initial consultation, your Inspire physician will look at the pump you currently have to see if there is a possibility of interaction between it and the Inspire system. As long as the two won’t interact, there are no issues to receive Inspire.
I have been through the whole sleep testing, given the Ccap, Claustrophobic. That was about a year ago. Will I have to go though the whole testing thing again. 🙁
My Husband is currently using a cpap. He has a lot allergy and sinus issues which the cpap seems to irritate. We are located in Louisiana and would like the name of a physician in our area.
Hi Cindi, To find a doctor near you, please visit InspireSleep.com/Doctor-Search/ and type in your zip code 🙂
I am 67 and by my sleep study test wake up 59 times an hour. Do I have to have a new sleep study test. I have not been found any mask that fits me due to my small face. If the mask is always too big and I can’t wear one without pulling the headgear straps so tight they are almost all the way velcro-ed to the back of my head! I wake up with DEEP gouges on my face and I only have a nasal mask. I have tried the pillows but they are too big for my nostrils. So I am left with the choice of this surgery which would be amazing if it worked. I am close to UVA in Charlottesville and wonder if Medicare will pay for it. Any suggestions on how or if this could happen?
Hi Sherrie, If your last sleep study is over 2-3 years old, you may need to get another one. Medicare is reimbursing for the cost of Inspire therapy based on medical necessity. Please visit InspireSleep.com/Doctor-Search/ and type in your zip code to find a doctor near you who offers Inspire therapy.
I have been diagnosed with severe sleep apnea last week and am meeting at the medical center in a few days to discuss my options. I tend to wake up once or twice per night to use the bathroom. If I have an Inspire implant would I have to deactivate it each time I wake and reactivate after use of the bathroom?
Hi Maxine, Inspire therapy features a pause button that will stop the therapy for 15 minutes during the night. Most people use this when they get up to use the bathroom, let the dog out, etc.
I had the Inspire device implanted about 13 month ago but unfortunately, it simply does not work for me. I was really excited at first (in fact I was the first person in Michigan to have the procedure done) because I believed that this would finally free me of CPAP and the nightly gluing of my lips together but that is not the case. I think that the Inspire concept is great and would work for someone who does not have problems staying asleep, but I wake up on my own every 30- 90 minutes and have to pause the device when I do. When the device comes out of pause, it comes screaming on at therapeutic levels and is like an alarm clock going off in my mouth, waking me back up. I’ve asked Inspire if they could implement a ramp mode to slowly bring the pulses back up to therapeutic levels over a short period of time (such that I would never even notice it came back on) but they tell me it is impossible. If I take the 10 mg of Ambien I can get through most of the night with Inspire but I really want off the sleeping pills so most nights I don’t even attempt to use it. In every case when I do use it, I always resort back to CPAP at some point in the night. They tell me that the next hardware level will address these things but that will hardly help me since I have the old one. So unfortunately this seems to be the end of the story for me; they tell me that there is nothing more that can be done and apparently given up on my case, I haven’t even had any follow-up from the team since last August. But I think my case is probably not typical and so I am still encouraging people to consider this therapy. Good luck and I hope it works well for you!
My heart goes out to you..
is it possible that they could give you the newer updated model ?
I just don’t understand why no fu..
have you tried reaching out to them per tc or by letter?
Ky
I had Inspire implant done June 2016. It has been a very bad experience. Many months just to get set up, due to Doctor and Inspire rep coordinating times. Trouble sleeping, zapping drives me crazy. Taking Ambien, still end up turning the device off after several hours. The generator keeps protruding outward sideways, apparently came unattached. I literally have to pop it back in place. I am getting intermittent pain near the rib incision. Doc said if I can live with these things he’s okay with it happening. Otherwise, surgery to reattach and rewire. The latest is to do an endoscopy to reprogram the electrodes in my throat so the zaps aren’t as bad. If I do this, I still have the other problems. Also, I feel bad all the time, tired, weak. Every, single day since surgery has been bad, I sometimes think it is slowly killing me. I want it removed at this point.
Inspire Therapy Team: It’s been over two and a half years since I had this implant and been unable to use it. Have you made any progress towards implementing a ramp mode so that myself and others can remain asleep when the device some out of pause mode? I’ve been requesting this since the first few months I had it implanted and all I get is silence.
Thanks,
Bill
They don’t even reply to my emails anymore when I ask (every 6 months) if there has been any progress towards resolving my issues.
I was diagnosed with severe sleep Apnea in 2003, 69 apnea’s per hour. Could not acclimate to CPAP under any mask or circumstances. Received the following surgeries to address very scarred tonsils and a nose that had been rebuilt a few times for full contact Tae Kwon Do, tonsillectomy and UPPP, rhino/septplasty. The surgeries improved my apnea but was still quite high and once again went through several CPAP machines and mask to no success. Tried the dental appliance nearly destroyed what was left of an already loose temporomandibular joint and causing frequent dislocations. Tried provents could not fall asleep. Also I am an insomniac anything not perfect at night keeps me awake. Noise, light, air blowing down my throat, difficult to exhale against the provents etc. Now there is Inspire Therapy which I am in the process of trying to get qualified for, however I just had a new baseline sleep study to find that 13 years later I’ve been diagnosed with 80 apneas/hour. The upper limit for Inspire is 65, so my question is why is there an upper limit, is because of irritation to the hypoglossal nerve or to the tongue? What are my chances of being approved? Also I wake up about 3 to 4 times a night to have urinate, it seems from another posting that Inspire HW is not suited for waking in the night as the reactivation from a pause comes on full blast not allowing one to fall back asleep, is this true? Help me out here I’m desparate going on 14 years with untreated severe sleep apnea, I’m 52 years old was working as an executive VP making a very good salary and had to quit my job last October as I could no longer keep up with such a demanding job on 90 minutes of sleep / night.
Hi Paul, Inspire therapy was tested in the STAR clinical trial in patients with an AHI of 20-65, which is where the numbers come from. If your sleep study is over 3 years old, an Inspire doctor will request a new one to get the most accurate sleep apnea severity reading. During the night, Inspire users can pause the therapy. This stops it for 15 minutes so they can use the bathroom, get a drink of water, let the dog out, etc. This is usually enough time for them to fall back asleep, then the therapy will begin again. Please visit InspireSleep.com to learn more, or send an email to [email protected].
Your need to urinate 3 – 4 times at night comes from your body attempt to counteract the high blood pressure caused by SOA. It causes a diuresis even if you are not taking a diuretic because you sleep apneas increase your blood pressure. If you reduce significantly your AHI ( through a CPAP’ Inspire or whatever) you will find out that you will need to get up only occasionally. That’s my experience, confirmed by research, unfortunately doctors don’t always communicate properly with patients.
Try not to drink water too late at night if possible
I am 25 years old and after my service in the Marine Corps, I found out that I have sleep apnea. I am in good shape and went through a sleep study stating I was waking up 72 per hour. I am concerned for the long term effects. I have a tonsil surgery which reduced my apnea to about 30 times per hour but am still concerned. The CPAP Mask does not work for me. I pull it off every night in my sleep and can’t go back to sleep after I am awoken. Is this a option I should be seriously considering?
Hi Lucas, If you are unable to get consistent benefit from CPAP, Inspire may be an option for you. Inspire therapy is on the Federal Supply Schedule, making it available for veterans such as yourself. Please visit our website to see a list of centers where Inspire is available.
I had mine put in recently with new remote. I wake up a few times with a very dry mouth. Any suggestions? Thanks
Hi Ron, Please send us an email to [email protected] to discuss further.
Why the limitation of BMI? My BMI is around 38. I am over weight due to lack of exercise which is directly affected by my severe OSA and having zero energy and little sleep. I have been on CPAP for over 10 years and able to tolerate but still extremely tired during the day.
Hi Hugh, Inspire therapy has not been tested in people with a Body Mass Index (BMI) of over 32. When people are overweight, they tend to have excess fatty tissue in their neck. When that fatty tissue is present, the therapy does not work. Your airway muscles can still be activated by the therapy, but the excess tissue will still block the airway and apnea events will still occur.
I’ve had 5 sleep studies in over 30 yrs. even when I weighed 89 lbs I had sleep apnea. I’m now at 150. Please please help me. Without good sleep, you don’t feel like doing anything. You are too tired. I worked for Social Service for over 30 yrs. please help. Mary Lou Peters…. and my first name is Mary Lou
Hi Mary Lou, If you are interested in Inspire therapy, you need to set up a consultation with an Inspire doctor. Please visit InspireSleep.com/Doctor-Search/ and type in your zip code to find a doctor near you.
Hi, I’m a mom with a 15 year old daughter who has Down Syndrome She has OSA and the next step is a Tracheotomy, but that will change our life and I want her to enjoy life I was online looking for other options before we go their.. Please help she cant make it though the nights without struggling.. I’m in Savannah Ga.
Hi Yolanda, Inspire therapy is currently available for people ages 22+. That being said, there is a clinical trial going on for down syndrome children who cannot use CPAP. Please visit https://clinicaltrials.gov/ct2/show/NCT02344108 to learn more about this trial.
I have tried everything including cpap, the oral device sleeping on my side provent, I can’t stand the clap as I am claustrophobic and tear it off every night and can’t get back to sleep after. I am in sales so I’m always tired and am on the road driving . Sometimes I even cancel my appts in morning as I’m so tired in the morning I have both apneas but my acl is only 14 and my bmi is 30 would I be a candidate for the inspire even though I am at a 13 or 14 when it says 20-65?? I look forward to hearing from you here soon. Thanks Pete
About 5 years ago I had a sleep study that showed that I only had 18 episodes per hour. Then, I went to an Inspire Ctr in Cleveland Ohio which received my test results from a different hospital in Akron, Ohio. The doctor told me that I did NOT qualify because I needed at least 30/hr! They should advertise this on the radio!
I am 48 have had severe OSA for at least 17 years. My BMI is 22 and I have never been over weight. I am looking into this as an option. I am a mouth breather, so can wear the nose pillows. The full CPAP is a mess. I’m still exhausted even when I do wear it. Many nights my machine gives me a sad face and says I did not have a good seal. I have tried many to get the right fit. Just cant do it. Not sure if an oral appliance would work. i was very excited about Inspire- but reading information concerns me. Hoping you can help. Sasy i can not lift weights even after healed? That is how I stay in shape, keep healthy and reduce stress. Also no MRI or diathermy. Some facial treatments make me wonder if they are diathermy. They are localized, not full body electromagnetic or high frequency. There are also some that are radio frequency (called Viora and Tru sculpt) the viora is more of my concern.
Hi Michelle,
Those are all great concerns. Please send an email to [email protected] or give us a call at 844-OSA-HELP to discuss them in depth 🙂
I have a pacemaker, would this prevent me from getting the INSPIRE device
If I qualify for the device and procedure, what would be my out of pocket costs for this device and procedure?
I am a mouth open breather. Would inspire therapy work for me?
I was wondering if upper airway stimulation addresses soft palate collapse as well as tongue base collapse cause I was researching and some sites say it addresses both and a couple sites I looked at said it only addresses tongue base collapse and my collapse is in the soft palate.
Was hoping to find an answer to your question, does Insoire work in both tongue base collapse and soft palate blocking upper airway in expiration. I have severe OSA, and am unable to get relief from soap due to soft palate closing when I exhale.
My oral device worked well for 8 years, but made my teeth loose and with chronic dry mouth, I’ve lost most of my teeth. I was in the process of getting implants to get back to the oral device, but I’ve been without a restorative nights sleep in 1 year. Help? Don’t want UPPP. To many failures and horror stories.
I am a sleep physician and have been working with ImThera Medical’s neurostimulation device, currently enrolling for our pivotal FDA trial. Both Imthera’s device and Inspire’s devices likely major mechanism of action is on the lateral walls of the throat and palate. If you need assistance as a patient, I am located in New York City.
May we view all comments from the installed base of patients?
Hi,
I have a diagnosis of moderate sleep apnea. I’m not a good candidate for CPAP, as I have chronic problems with allergies and my sinuses, have had a UPPP, and my skin is sensitive to the mask materials. I would like to know if I would be a candidate for the implant. My BMI is normal, but the imaging test shows that I have a small airway. Would that automatically disqualify me from this treatment option?
Thank you,
Karen
Hi my name is Lori I have server sleep apena .My number is 150 a hour . I also have a bundle branch blockage on my left side . My question is will that stop me from getting the device ?I have been on CPAP for about 4 yrs .
Thanks very good information. signifcant support.
Hi. I’m 64 and my last sleep study ( home) in 2014 indicated I have OSA with 54 AHI. My AHI had increased over the last 20 yrs of diagnosed OSA. I’m in good shape other wise with a BMI of 25. I tried many procedures to improve my OSA – CPAP ( cant tolerate ), UPPP, nasal septum/turbinate repairs, HF ablation of the base of my tongue and an oral appliance for 2 years. A large tongue seems to be my major issue. I’m about to take another HOME sleep study to quantify the improvement with my oral appliance. 3 sleep studies in a lab have ended in 3- 4 hrs, as I cant get back to sleep with 40 wires attached to my head, torso, legs etc. Could Inspire be a possibilty for me ???
Why is it that the only reviews from actual people with the device implanted that I can find, outside of the inspire’s website, are negative? Dry mouth, can’t sleep because of the zaps, tongue problems, etc. Real user comments are only slipped into forums like this (buried deep) – a huge cause for concern. It seems like the company is hiding a lot of real testimonials. I was interested as I’m a “good candidate”, but until there’s more evidence not hand picked by inspire I’ll wait.
Hi Kevin, I just completed the procedure. I will be happy to provide my candid experience. My wife is in the dental field and was very reluctant to let me proceed.
Kevin, I was part of the STAR trial in 2011. I am going on 7 years with it and it is wonderful! Only problem I had was tongue abrasions from my tongue moving forward over my teeth. I heard that has been taken care of because of the trial I was in. They adjusted the placement of the lead on the nerve so that doesn’t happen. If you are a mouth breather you will always have dry mouth no matter what. I sleep and feel wonderful as long as I remember to turn it on before going to sleep.
I have an appointment for inspire. Is their a weight limit I weigh 400lbs. I tried clap but Im closterfobia.
Will I be able to get this device if I have a heart pacemaker?
I have a duel lead pacemaker. I also am a diabetic.
I am 5’6 and weigh 160 lbs. I was first diagnosed with OSA some 15 yrs. ago and was put on a c-pap
machine which I could not tolerate. I then had surgery to remove some of the soft tissue and the
uvula which was successful and free of sleep apnea
until a year ago when my sleep apnea returned and
again started on a c-pap. Am I a candidate for you?
Because of other health problems, I am likely to have one or more MRIs in the future. Is it possible for me to have this Inspire therapy implant and still get an MRI? How about X-rays?
Following this, as i need yearly central nervous system MRIs due to my MS
Hi Dean, in the beginning of the article they said if you are going to have an mri that the unit would have to be removed. I’m not a pro I’m just letting you know what o understand.
What is the weight limit for the Inspire Therapy?
Thank you
I am a sleep apnea patient. Upon reading I found that this new device is very helpful. I would like to know who qualifies and does Tricare And Medicar pay for it?
I am curious why this procedure is being limited to patients with just obstructive sleep apnea? Why not for central sleep apnea? Same outcome, but different causative factor.
would this procedure be good for me? have a pacemaker
I am 5’3 185 disqualified for not being 180 .Tough pill to swallow.FDA make it to hard how can you better yourself.
I would think twice—no, make that three times—before implanting the Inspire device. I wish I had. Immediately after one was implanted in me last summer, most of my body shut down. I was in critical condition with acute pulmonary edema and many other serious problems. I was on a respirator for about a week and in the ICU for three weeks. After-effects continued for at least five months. I missed work for the entire second half of 2019. Implanting the Inspire device is not the minor out-patient procedure that the company suggests. Beware.
any other issues like nausea vomiting
Hi,
Does kaiser have docs performing inspire surgeries?
I am interested. I know there is a BMI requirement and was wondering if there was an age limit. I am 75 years old and I’m good health. Thank you b for your reply. Anne Downey
Does Inspire work for central sleep apnoea?