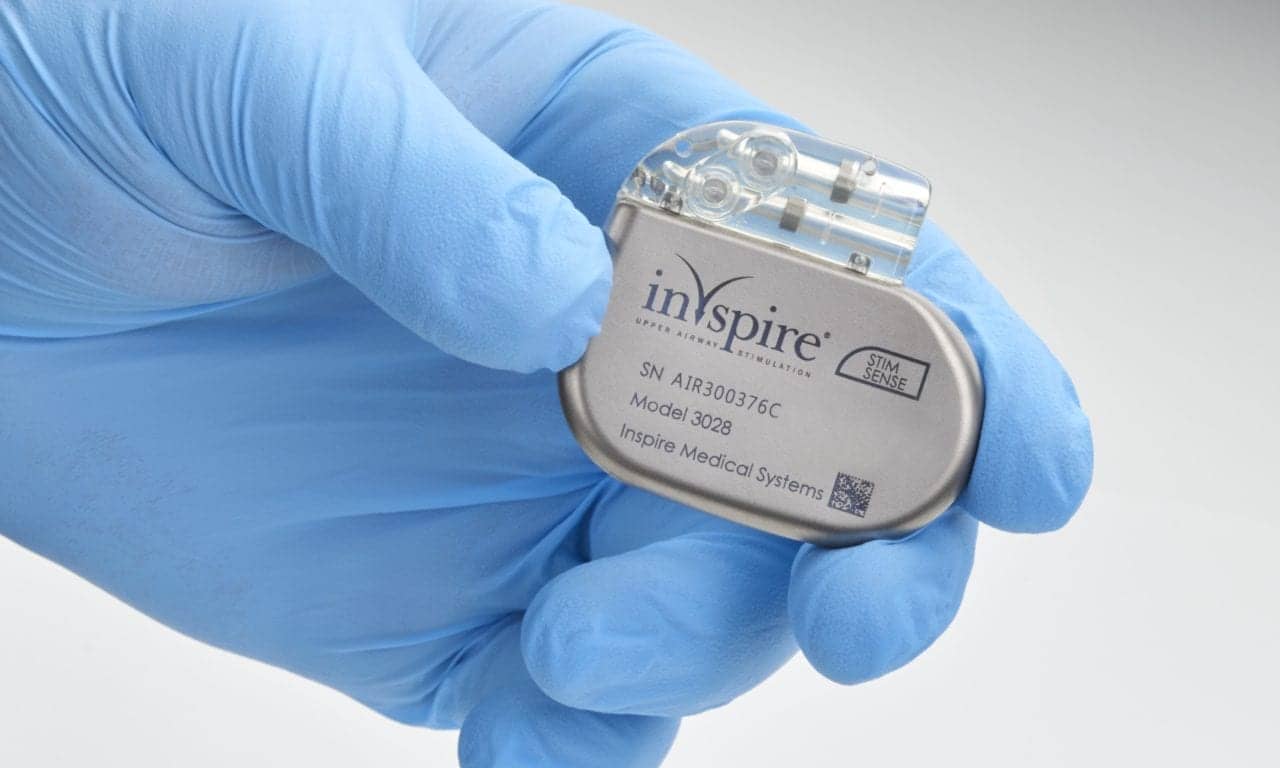
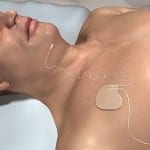
Over 60,000 Patients Treated With Inspire Therapy for Sleep Apnea
Inspire Medical reported in its fourth-quarter earnings that it surpassed 60,000 patients treated with its Inspire neurostimulation therapy for obstructive sleep apnea as of the end of 2023.