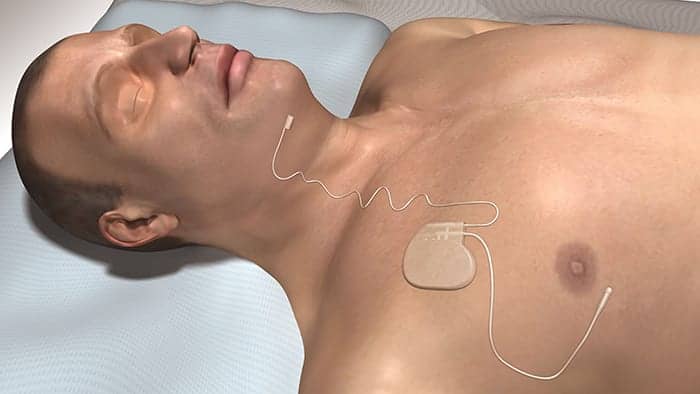
The United States Food and Drug Administration (FDA) has granted Inspire Medical Inc Breakthrough Device Designation for a potential increase in the upper limit of apnea hypopnea index (AHI) and body mass index (BMI) for patients to be eligible for Inspire Upper Airway Stimulation for obstructive sleep apnea.
The FDA’s Breakthrough Therapy program was created to help patients and healthcare providers receive faster access to innovative technologies that hold the potential to provide more effective treatment of irreversibly debilitating diseases or conditions.
“We plan to submit to the FDA our request to increase Inspire therapy’s indication by expanding the upper limit of AHI to 100 events per hour from 65, as well as raising the BMI warning in our indication to 40 from 32,” says Tim Herbert, president and CEO of Inspire Medical, in a release. “Our application for this expanded indication recently received Breakthrough Device Designation from the FDA, thereby reducing the review time.”
In other Inspire news, the FDA recently gave Inspire approval for new stimulation and sensing silicone leads, which Herbert says will provide improved manufacturability, easier system implantation, increased long-term performance, and enhanced reliability. “We are targeting the US launch of the new leads for later in 2022,” he says.
Inspire will also soon be executing the full US launch of its new Bluetooth-enabled patient remote, an important aspect of its SleepSync program.
Its international business also continues to gain momentum. “In Europe, Germany benefited from a strong rebound in procedure growth in the second quarter, and we expect to maintain this progress throughout the second half of the year. Therapy adoption growth also continues in the Netherlands and in the United Kingdom, and we have made significant reimbursement advancements in France, Belgium, and Austria. In Asia, additional centers in Japan have conducted their first Inspire procedures, and initial Inspire cases have been performed at two separate centers in Singapore,” Herbert says.
Inspire is maintaining its guidance relating to the opening of new US medical centers of 52 to 56 per quarter for the remainder of the year, as well as its guidance of 11 to 12 new territories per quarter for the remainder of the year.