4-Year-Old with Down Syndrome and OSA Benefits from Hypoglossal Nerve Stimulation: Case Study
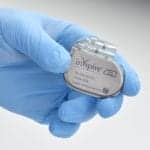
The patient experienced a 40% decrease in the apnea-hypopnea index after receiving Inspire, currently approved for children with Down syndrome and OSA over age 13.