What MRI compatibility means for neurostimulator referrals for sleep apnea.
By Sree Roy
Sleep specialists and patients consider many variables when deciding whether an implanted neurostimulator is a sleep apnea therapy road they want to embark upon. The past year has brought relief that one bump has now been smoothed down: magnetic resonance imaging (MRI) compatibility.
“I wouldn’t put [MRI compatibility] high on my list of concerns, but it definitely is a variable that many of our patients consider in their decision-making,” says neurologist-sleep specialist Asim Roy, MD, medical director of the Ohio Sleep Medicine Institute. “Now that Inspire and remedē are MRI-compatible, it’s just one less thing to worry about, which is a huge win.”
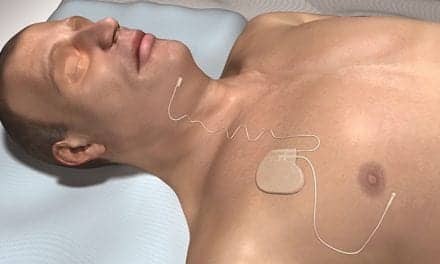
The US Food and Drug Administration (FDA) granted the Inspire Model 3028 (also known as Inspire IV) neurostimulator for obstructive sleep apnea full-body 1.5T MRI conditional approval in July 2022, expanding on its previous labeling that allowed only head, neck, and extremity MRIs. In March, the FDA granted ZOLL Respicardia MRI full-body compatibility for all models of the remedē System for central sleep apnea. The FDA has also already granted the investigational Genio neurostimulator by Nyxoah SA 1.5T and 3T conditional full-body MRI compatibility, which means that upon FDA approval (which could be as early as the fourth quarter of 2024), Nyxoah’s Genio neurostimulator for obstructive sleep apnea will be MRI-compatible out of the gate.
To be sure, explants due to lack of MRI compatibility are rare. According to an Inspire Medical Systems spokesperson, the explant rate in 2021 across all Inspire devices was less than 1%, of which MRI incompatibility was a subset.
Explants are rare in part due to sleep specialists and surgeons considering patients’ future MRI needs prior to device implantation. “My recommendation to surgeons who implant is, if the patient has a high-risk disease, to act as part of a patient care team and discuss with the providers who have more knowledge and insight in needs for specialized MRI or other testing,” says sleep specialist-sleep surgeon B. Tucker Woodson, MD, who, in almost a decade of neurostimulator implantations, has done only two explants due to lack of MRI compatibility.
Even with advances in MRI compatibility for neurostimulators, Woodson continues to advise patients that “there is a small risk that you would need an examination using newer, more powerful technology that is not compatible…and that it might require removal.”
That is because MRI technology will likely continue to improve and become more powerful, and to date, there is no surrogate for an MRI. CAT scans are a “distant second,” explains Roy, “but they don’t image soft tissue very well.” So implanted neurostimulator makers must provide evidence to the FDA of how their device performs at various magnetic field strengths, scan times, wait times, and more—alleviating concerns about the potential for heat-related injuries.
ZOLL Respicardia’s vice president of global marketing, Seamus Jackson, MBA, says, “The MRI conditional approval was granted after we successfully completed robust MRI compatibility testing and submitted for review by the FDA. Because there were no changes to the system itself, patients who already have remedē devices are included in the MRI conditional approval.”
At Nyxoah, full-body 1.5T and 3T MRI compatibility were defined as “must-haves,” according to Rémi Renard, Nyxoah’s vice president of marketing and market development. “No patient should lose the chance of an optimal diagnosis of his/her pathology or require device explant due to MRI compatibility restrictions,” he says.
According to sleep specialists, most patients find that 1.5T MRI conditional approval for a neurostimulator is adequate to remove MRI compatibility as a potential obstacle. “For most patients, as long as they still have the ability to enter the scanner, their concerns are largely alleviated,” says sleep specialist-sleep surgeon David Kent, MD, assistant professor of otolaryngology at Vanderbilt University Medical Center. “I’ve had very few patients who’ve expressed concern regarding the particularities of magnetic strength and imaging parameters.”
To communicate safe MRI conditions to radiology teams, neurostimulator manufacturers post their MRI guidelines on their websites, as well as staff phone hotlines.
At Inspire, a spokesperson says the most common questions its radiology hotline receives are 1) MRI guidance—general questions about its manual and scan conditions, and 2) verifying patient information—confirming with patient services their patient has Inspire and verifying MRI eligibility.
Kent says, “In my own experience, MRI technologists are quite fastidious about determining what foreign bodies exist within a patient before entering a scanner and what the constraints are regarding possible imaging. My office has been contacted many times in the past by radiology teams who are not 100% confident regarding MRI safety prior to an exam.”
While recent MRI-compatibility approvals probably won’t impact the explant rate for existing users, sleep specialists expect the change to push the door further ajar for new neurostimulator candidates.
Photo 29602723 © Sudok1 | Dreamstime.com