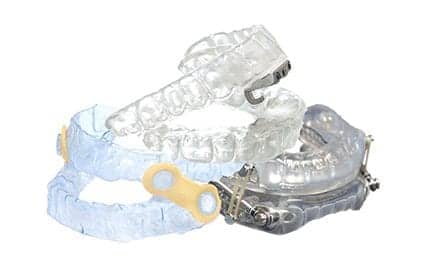
Vivos receives the FDA’s first clearance for oral appliances to treat severe OSA, while ProSomnus accelerates its regulatory submission for a severe indication. A look at how these devices work, their efficacy, and what it means for patient care.
By Alyx Arnett
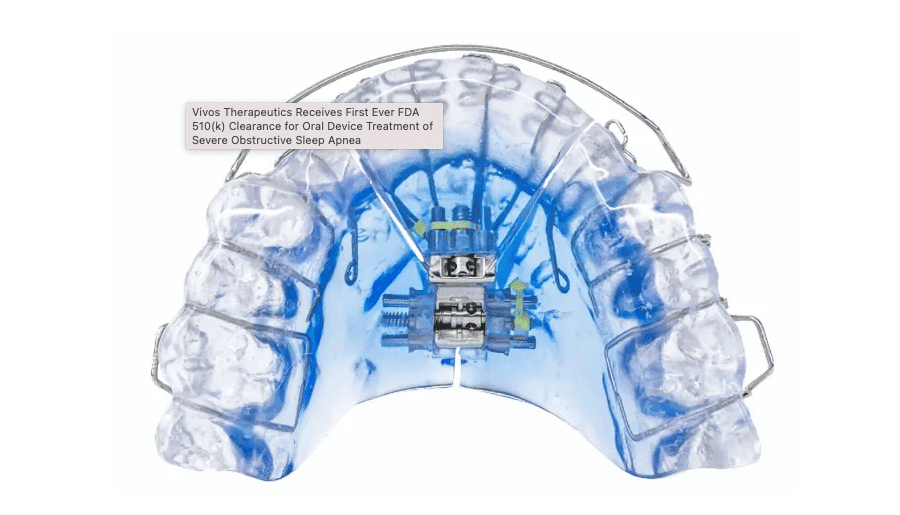
The US Food and Drug Administration (FDA) recently expanded the indications for use of a line of oral appliances manufactured by Vivos Therapeutics, adding that the devices are indicated for the treatment of moderate and severe obstructive sleep apnea (OSA) in adults—representing the first time the FDA has granted an oral appliance clearance to treat severe OSA.
The 510(k) clearance covers Vivos’ removable Complete Airway Repositioning and/or Expansion (CARE) oral appliances, which include the company’s flagship day and night appliance (DNA), the mandibular-repositioning nighttime appliance (mRNA), and the modified mandibular repositioning nighttime appliance (mmRNA).
“By adding the word ‘severe,’ we can potentially help and treat so many more people that weren’t able to have that option,” says Scott Simonetti, DDS, vice president of research and development at Vivos and founder and CEO of Advanced Facialdontics, an oral orthotics company recently acquired by Vivos.
The CARE appliances consist of an upper and lower tray, which put pressure on the back of the throat to prevent the airway from collapsing during sleep. The DNA appliance does not connect the upper and lower trays (no mandibular advancement), while the mRNA and mmRNA appliances connect the trays with a flange and hinge, respectively.
The devices are cleared for severe sleep apnea on their own or along with positive airway pressure (PAP) and/or myofunctional therapy, as needed. According to Colette Cozean, PhD, regulatory consultant for Vivos, who did the data assessment and analysis and filing with the FDA, the “as needed” designation allows for situations, for instance, in which a patient exhibits very severe clinical symptoms, and their healthcare provider may opt to prescribe PAP therapy initially and continue it as required.
“If they’re using an mRNA, they already have mandibular advancement, and they probably would not do that. But if they’re using DNA, they might use PAP to be sure that the patient’s clinical symptoms are not a problem at the beginning” of therapy, says Cozean. “Or, if you want to train the tongue on where to go, you might use myofunctional therapy.”
Sleep physician David E. McCarty, MD, FAASM, owner and CEO of Empowered Sleep Apnea, views this pathway as a significant shift in recognizing the complex aspects of OSA. “This really embraces a pathway that has multiple layers at the discretion of the provider, so it encourages a culture to start examining this problem like an engineer and recognizing the many moving parts,” McCarty says. “That’s a complete change of mindset, and that’s why this is such an important decision.”
Vivos data submitted to the FDA from 73 severe OSA patients showed that 80% experienced an improvement of at least one classification or at least a 50% improvement in the apnea-hypopnea index (AHI). Thirty-seven were treated with a CARE appliance as a standalone therapy, resulting in an AHI improvement of 52.8%.
After receiving the expanded indication for severe OSA in November, Vivos reported an uptick in interest for the fourth quarter of 2023, with preliminary metrics showing a 600% increase in new dentist inquiries and signed dentist enrollment contracts up 38% sequentially over the third quarter.
Lasting Improvement in Airway Function
Vivos’ CARE appliances are designed to expand the nasal airway through jaw expansion and mid-facial redevelopment, potentially offering a permanent improvement to the oropharyngeal airway without requiring lifelong treatment.
The maxillary portion of the devices consists of polymer subunits with embedded metallic coils designed to increase airway volume over time. The DNA appliance can be expanded in the anterior-posterior and lateral dimensions. Patients wear the appliances nightly (the DNA appliance can also be worn throughout the day) and adjust them during use to gradually expand them and allow maxillary expansion.
According to Vivos, the therapy is typically completed within 18 months, though many patients complete it earlier. Data submitted to the FDA showed the average treatment time was 9.7 months. In a separate retrospective study of 220 patients who underwent CARE, 25.9% had a complete resolution of OSA symptoms.1 The median length of therapy was 13 months, with a daily wear time of 12 hours.
All pre- and post-treatment testing was conducted with no device in the mouth—an “interesting and important” factor, according to Clete Kushida, MD, PhD, FAASM, a co-author of the retrospective study and Stanford Sleep Medicine’s division chief and medical director.
Conventional oral appliances typically remain in the patients’ mouths when post-treatment measures, such as the AHI derived from sleep studies, are obtained, he says. “These non-permanent, removable appliances are able to modify the upper airway in such a way that the appliances achieve positive effects without having to remain in the mouths of patients,” says Kushida, also associate chair of sleep medicine and a professor in the department of psychiatry and behavioral sciences at Stanford University Medical Center, as well as director of the Stanford Human Sleep Research Laboratory.
A study presented at CHEST 2023 evaluating severe versus moderate OSA patients showed results were slightly better for patients with DNA versus mRNA, indicating that the primary improvement mechanism may be maxillary expansion.2 “We are currently exploring the precise anatomic mechanisms by which we believe these devices achieve lasting effects without them in place in the patients’ mouths,” he says.
ProSomnus Pursues Severe Indication Clearance
Following Vivos’ clearance, ProSomnus Sleep Technologies is accelerating its FDA submission for a severe indication for its EVO Sleep and Snore Device, which is currently in a clinical trial, the Severe OSA Study.
ProSomnus’ submission will rely on clinical data from previous prospective clinical studies. Meanwhile, the Severe OSA Study will continue as planned, according to Erin Mosca, PhD, director of scientific and medical affairs at ProSomnus, who is working on the study.
One prospective study, the First Line Obstructive Sleep Apnea Treatment (FLOSAT) study comparing the effectiveness of the EVO device—intended to reduce snoring and OSA via mandibular repositioning—to CPAP therapy, showed the oral appliances were non-inferior to CPAP for the treatment of moderate to severe OSA.

The EVO device treated 79% of exclusively severe OSA patients, using the same efficacy criteria as data reported for hypoglossal nerve stimulation: an AHI of less than 20 and a 50% improvement. In comparison, neurostimulators, like those in the STAR trial, showed a success rate of around 66% in patients with moderate to severe OSA. ProSomnus now intends to design a clinical trial to compare its oral appliances to hypoglossal nerve stimulation for the treatment of severe OSA.
FLOSAT also found that 98% of patients continued oral appliance therapy at three months, versus 22% discontinuing CPAP therapy over the same period.
Additionally, a pilot study of EVO devices for the treatment of OSA, published in the journal Cureus in December, found that 80% of patients with severe OSA, without screening or excluding subjects for airway collapse profile, were treated to an AHI of less than 20 with a 50% improvement in AHI.3
“We’re in the midst of petitioning the FDA to say we’d like to submit data we have already,” says Mark Murphy, DDS, DABDSM, lead clinical faculty for ProSomnus and owner of dental sleep practice Funktional Sleep in Rochester Hills, Mich.
According to ProSomnus’ Mosca, further data from the Severe OSA Study will be “crucial in our efforts to have oral appliance therapy accepted as a treatment for severe OSA by professional societies.” To date, 25 individuals have been enrolled in the study, and Mosca anticipates the study will be fully enrolled by the end of 2024.
Other oral appliances also have demonstrated effectiveness in treating severe OSA. For instance, in a study of CPAP-resistant cases, Dream System’s OASYS Oral/Nasal Airway System resolved 46% of severe cases with a post-treatment AHI of less than 5, while 66% had an AHI of less than 10. For very severe cases, 43% were resolved with a post-treatment AHI of less than 5, while 64% had an AHI of less than 10.4
Path to Acceptance as a First-Line Treatment
The American Academy of Sleep Medicine and the American Academy of Dental Sleep Medicine’s 2015 clinical practice guideline recommends sleep physicians consider oral appliances for adult OSA patients who are intolerant of CPAP or prefer alternate therapy, while it maintains that CPAP remains the primary treatment option.5
Neil Verdal-Austin, CEO and managing director of oral appliance manufacturer SomnoMed, says the oral appliance clearance for severe sleep apnea is “positive for the industry as a whole.”
Stanford’s Kushida, who served as chair of the Standards of Practice Committee of the American Academy of Sleep Medicine from 2003-2005 and led the 2005 update on practice parameters, foresees a significant shift.
“I believe now that oral appliances will no longer be relegated to second-line treatment for OSA, that oral appliances and orthodontic treatments will be more frequently used to treat patients with OSA, including those with severe cases, and that the field of dental sleep medicine will become even more prominent in the future,” Kushida says.
But Kent Smith, DDS, DABDSM, ASBA, CEO of Star Sleep and Wellness, believes significant changes will be slow. Establishing further credibility would require peer-reviewed double-blinded studies and real-life, sequential before-and-after sleep studies demonstrating success, along with “a massive paradigm shift” in prescribing physicians and a change in the protocols of insurance companies, he says.
Vivos’ Simonetti also believes that more extensive trials with control groups, designed to capture clinical outcomes of interest to medical doctors, are crucial for wider acceptance and implementation. While Empowered Sleep Apnea’s McCarty also agrees that a study design that allows more generalizability would help, he points out: “Absence of data doesn’t necessarily mean absence of signal.”
McCarty says, “The tendency on the Western medical side is to belittle approaches that don’t have the randomized control trial behind them when, in reality, we should be paying attention to the bright spots and helping to innovate those projects that are showing promise to greater scientific scrutiny rather than create barriers to development.”
Regarding shifts in insurance protocols, some changes are underway. UnitedHealthcare, for example, recently updated its medical policy. Starting March 1, the insurer will require an oral appliance trial before approving sleep surgeries—such as hypoglossal nerve stimulation, uvulopalatopharyngoplasty, mandibular osteotomy, and maxillomandibular osteotomy and advancement—for patients with moderate and severe OSA.
Looking ahead, ProSomnus’ Mosca envisions a future where, once the medical community recognizes the safety and efficacy of oral appliance therapy, it will become a primary treatment option for all severities of OSA. “Given that oral appliance therapy is very well tolerated and preferred to other therapies by many patients, the result will be more individuals with OSA being effectively treated by their OSA therapy,” Mosca says.
Marc Morisset, vice president of oral appliance maker Panthera Dental’s sleep division, says, “All things considered, when patients are adequately treated for OSA, not only do patients benefit, but healthcare cost burden declines, and patients achieve improved healthcare outcomes.”
References
- Katz D, DeMaria S, Heckman S, et al. Use of the complete airway repositioning and expansion (CARE) approach in 220 patients with obstructive sleep apnea (OSA): a retrospective cohort study. Sleep Med. 2022;99:18-22.
- Kushida C, Cozean C, Alexander J. Treatment of severe sleep apnea by novel oral appliances. CHEST. 2023 Oct. 164(4)suppl:A6288-9.
- Sall E, Smith K, Desai A, et al. Evaluating the clinical performance of a novel, precision oral appliance therapy medical device made wholly from a medical grade class VI material for the treatment of obstructive sleep apnea. Cureus. 2023 Dec 7;15(12):e50107.
- Shrivastava D, Bixby JK, Livornese DS, et al. Efficacy of oral appliance therapy in the treatment of severe OSA in CPAP-resistant cases. Sleep Vigil. 2018;2:119-25.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015 Jul 15;11(7):773-827.
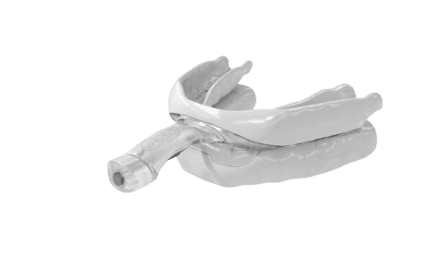
Photo caption: Vivos’ mmRNA oral appliance
Photo credit: Vivos