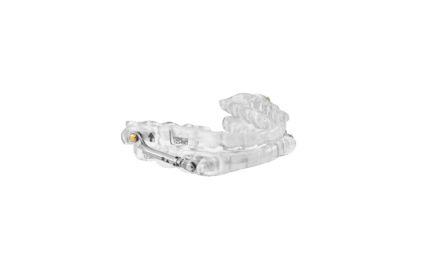
Vivos, a medical technology company focused on developing and commercializing innovative diagnostic and treatment modalities for patients suffering from mild to moderate obstructive sleep apnea (OSA) and snoring, announced that it has received acceptance from a Centers for Medicare and Medicaid Services Pricing, Data Analysis and Coding (“PDAC”) contractor for its mmRNA (modified mandibular Repositioning Nighttime Appliance) device for treating mild to moderate OSA and snoring in adults.
This acceptance places the mmRNA device on the PDAC list of oral appliances covered by and billable to Medicare. This development immediately makes benefits of the mmRNA device available to millions of Medicare beneficiaries who seek effective treatment for mild to moderate OSA.
Acceptance on the PDAC list marks a positive leap toward increasing commercial insurance coverage, making the mmRNA appliance a cost-effective solution for non-Medicare beneficiaries. With its novel design, the mmRNA appliance also allows Vivos-trained dentists to help patients in a variety of clinical applications, such as a standalone therapy for mild and moderate OSA or as an alternative to or an adjunctive therapy with continuous positive airway pressure (CPAP) therapy.
“This latest regulatory milestone opens the door for Vivos-trained dentists across the country to deliver our life-changing therapeutic technology to the millions of OSA sufferers in the US who are either on Medicare or who have commercial medical insurance coverage that follows Medicare guidelines,” says Kirk Huntsman, Vivos CEO and chairman, in a statement. “The latest data from our SleepImage home sleep apnea test screening shows that 47% of patients screened were positive for OSA, a far higher prevalence of OSA than has previously been assumed in the industry.”




![5 Steps to Oral Appliance Therapy [Podcast]](https://sleepreviewmag.com/wp-content/uploads/2019/02/oral-appliance-steps-440x264.jpg)