Today, the US Food and Drug Administration (FDA) authorized marketing of a new prescription only device intended to reduce snoring and mild obstructive sleep apnea (OSA). Unlike devices used while patients sleep, this is the first device used while awake that is intended to improve tongue muscle function, which in time can help prevent the tongue from collapsing backwards and obstructing the airway during sleep.
“Obstructive sleep apnea not only impacts sleep quality but can have other serious health impacts if untreated. Today’s authorization offers a new option for the thousands of individuals who experience snoring or mild sleep apnea,” says Malvina Eydelman, MD, director of the Office of Ophthalmic, Anesthesia, Respiratory, ENT and Dental Devices in the FDA’s Center for Devices and Radiological Health, in a release.
Mild OSA is defined by the FDA as an apnea-hypopnea score of more than 5 but less than 15. The device, the eXciteOSA, is a removable tongue muscle stimulation device that delivers neuromuscular stimulation to the tongue to reduce snoring and mild sleep apnea for patients who are 18 years or older.
[RELATED: Sleep Apnea Device Developer Signifier Closes $10M Series C Round]
The eXciteOSA device works by delivering electrical muscle stimulation through a mouthpiece that sits around the tongue. The eXciteOSA mouthpiece has four electrodes, two located above the tongue and two located below the tongue. The device provides electrical muscle stimulation action in sessions that consist of a series of electrical pulses with rest periods in between. It is used for 20 minutes once a day during a wakeful state, for a period of 6‐weeks, and once a week thereafter.
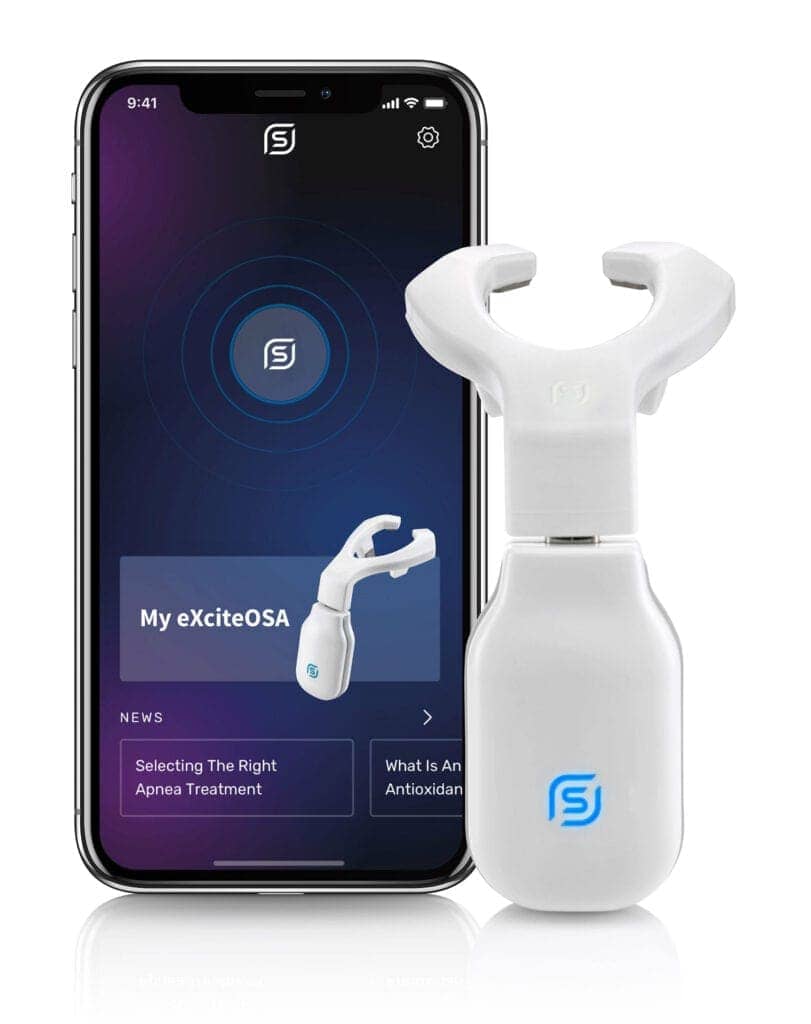
The FDA assessed the safety and effectiveness of the eXciteOSA device in 115 patients with snoring, including 48 patients with snoring and mild sleep apnea. All patients used the device for 20 minutes, once a day for 6 weeks, then discontinued use for 2 weeks before they were reassessed. Overall, the percent of time spent snoring at levels louder than 40dB was reduced by more than 20% in 87 out of the 115 patients. In a 48-patient subset with snoring and mild OSA, the average AHI reduced by 48%, from 10.21 to 5.27, in 41 out of 48 patients. The most common adverse events observed were excessive salivation, tongue or tooth discomfort, tongue tingling, dental filling sensitivity, metallic taste, gagging, and tight jaw.
Patients should receive a comprehensive dental examination prior to use of the device. The eXciteOSA device is contraindicated for patients with pacemakers or implanted pacing leads (electrodes); patients with temporary or permanent implants, dental braces, intraoral metal prosthesis/restorations/appliances, or dental jewelry in the mouth; patients who are pregnant or may be pregnant; or patients who have ulcerations in or around the mouth. The eXciteOSA device is not intended for patients who have or are suspected of having OSA with an AHI of 15 and higher.
The FDA reviewed the device through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type. Along with this authorization, the FDA is establishing special controls for devices of this type, including requirements related to labeling and performance testing. This means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) premarket notification process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device. When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type.
The FDA granted the marketing authorization to Signifier Medical Technologies LLC.
Photo 67647407 © Catalin205 – Dreamstime.com




Can’t wait to see how many lawyers line up behind this one to file lawsuits. A little common sense; a patient has been told they have OSA and quit breathing 14 times per hour for 10 seconds or longer. Does not go on PAP therapy but uses this. Has a cardiac or stroke event during sleep. Sounds like DMD’s are practicing medicine without a legal liability safety net. I would get serious legal counsel and talk to your malpractice insurance company before I gave this to someone.
Calm down!! Jesus, people like you prevent disruption, bet you are one of those old school – who feel that you are left out.
Thus is a prescription only!!
Have you seen their clinical data? And the possibility of PAP compliance in Mild OSA patients are next to nothing.
Please use your common sense before commenting.
I would tend to agree with SleepyTyme with one addition. You mention PAP therapy as the only treatment for OSA with a AI of 14.
I would add a mandibular advancement device as another possible treatment option.
The eXcite OSA device is DAY TIME treatment only. The great thing about the device is – it doesn’t interfere with NIGHT TIME “old school” products like PAP and MAD.
James, are you suggesting MAD and PAP together??? Wow? On a patient with Mild OSA?
I would really reconsider that and ask patients what do they want.
Let’s make sure we get all the details straight. While this wonder gizmo is surely very attractive, do know the investment you’re in for. Firstly, is NOT covered by your insurance, so you will pay out of pocket 100% no pay-plans. Secondly, the mouthpiece stops working after 90 days. So, you’ll need a new one every 90 days/3months for the wonderful price of $90. Be ready to make sure you are okay with this business model and be a cow for their milking pleasure for the future.
I’d be concerned about blasting my head with Blue Tooth radiation and/or the upcoming dangerous 5G. Talk about law suits. They need to develop a safer technology.