 |
Trained to identify typical obstructive and central sleep apnea phenotypes of SDB, many sleep lab clinicians and technicians remain unfamiliar with complex sleep apnea syndrome (complex SAS). This syndrome can be considered the simultaneous presence of upper airway obstruction and chemoreflex-driven respiratory oscillations during sleep. Complex SAS is often encountered in the clinical sleep laboratory. The complex-pattern breathing of predominantly mixed apneas and obstructed periodic breathing in some patients, or obstructive apneas that resolve with CPAP only to reveal central apnea or the Cheyne-Stokes breathing pattern, is not classified as a distinct breathing disorder.1-3 Its prevalence ranges from 15%1 to as high as 48%4 in clinical practice, based on study population characteristics. The difficulty in recognition may be directly related to the standard practice in sleep labs of not scoring mixed apneas and central hypopneas, with limitations of viewing the PSG in 30-second epochs and the scoring guidelines governing it.5 Enhancing the knowledge base of SDB phenotypes, creating greater awareness of complex SAS among sleep lab clinicians and technicians, and defining complex SAS in objective terms that are extracted from the current literature help clarify some of the complicated nature of complex SAS.
COMPLEX SAS PATIENT CHARACTERISTICS
Only two measures discriminate complex SAS patients from obstructive sleep apnea (OSA) and central sleep apnea (CSA) patients.1 Research findings show the male sex was more predominant in complex SAS than OSA and CSA, and complex SAS patients had less sleep maintenance insomnia than OSA and CSA patients. No other patient characteristics were statistically significant to distinguish complex SAS from OSA and CSA patients (see Table 1).
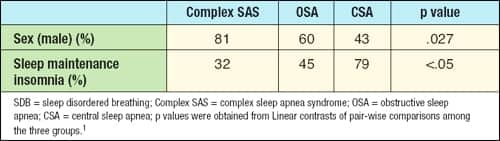 |
| Table 1. Significant patient characteristics of SDB phenotypes. |
COMPLEX SAS PHENOTYPIC PSG FEATURES
Two types of complex SAS have been identified, hypocapnic and hypercapnic. The hypercapnic type consists of congenital hypoventilation, chronic obstructive lung disease, obesity hypoventilation, etc. Several identifiable variables linked to the hypocapnic variety of complex SAS have been reported by Gilmartin et al (see Table 2).6
SDB
Complex SAS patients present with relatively short-cycle mixed types of apneas (repetitive, ~ every 25-30 seconds) rather than pure obstructive or central apneas on the PSG.7 The hypopneas are obstructive but oscillate similar to periodic breathing—waxing and waning symmetrically. Even the obstructive apneas may oscillate with a pattern resembling periodic breathing.8 Research finds that complex SAS patients had a statistically significant higher non-rapid eye movement (NREM) sleep obstructive AHI than CSA and OSA patients and total obstructive AHI1 (see Figure 1). It is not known what minimum number of mixed apneas or obstructed periodic breathing indicates complex SAS in SDB patients.
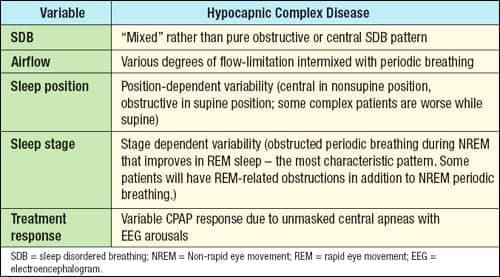 |
| Table 2. Hypocapnic complex disease characteristics in sleep. |
Sleep Position
Varying body sleeping position from supine to side, for example, elicits a change from obstructive to central type of SDB events suggesting complex SAS. The reverse scenario is also possible. Often, CSA is induced following stabilization of the upper airway with CPAP.9 Morgenthaler et al found that patients with complex SAS on CPAP had a significantly higher total mean arousal index (AI) compared to OSA patients but were similar compared to CSA patients.1 The respiratory-related AI with CPAP was significantly higher in complex SAS compared to OSA and similar to CSA patients. CPAP did not resolve SDB in complex SAS and CSA patients (see Figure 2). The CPAP findings in patients with CSA are comparable to another study by Teschler et al using a full night PSG.7
Sleep Stage
Periodic breathing observed during unstable NREM sleep and severe obstructive events with corresponding oxygen desaturations in REM sleep suggest the presence of complex SAS. This variability is easily seen in the first half of the night with predominant unstable NREM periodic breathing and in the second half of the night when REM sleep is more prevalent. The more prevailing SDB may be severe obstructive sleep apnea.
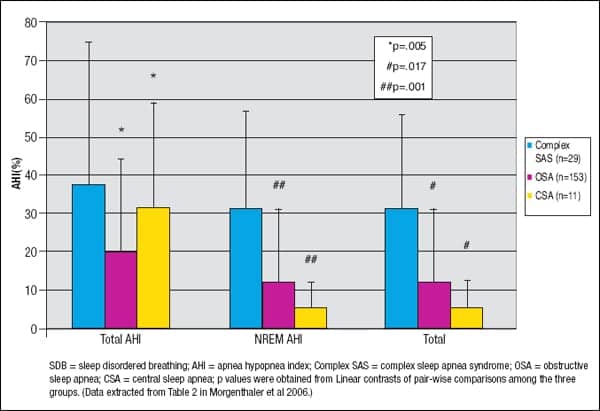 |
| Figure 1. Comparison of Mean (± SD) Total Apnea Hypopnea Index, NREM Apnea Hypopnea Index, and Total Obstructive Apnea Hypopnea Index in complex SAS, OSA, and CSA phenotypes. |
Treatment Response
A more subtle form of complex SAS may present with minimal SDB events in REM sleep—periodic short cycles of obstruction during unstable NREM sleep and incomplete tolerance to positive airway pressure.6 Some noncompliers to CPAP therapy may fall into this category.
EMPLOYING TECHNOLOGY
Cardiopulmonary coupling (CPC) analysis is an automated measure of cardiopulmonary interactions during sleep using a single-channel electrocardiogram (ECG). This technique combines heart rate variability and ECG-derived respiration (the R-wave amplitude fluctuations induced by respiration) to generate the sleep spectrogram.4 Following artifact extraction and data resampling, the cross-power and coherence between both cardiac and respiratory signals are quantified to yield the ratio of coherent cross-power in the low-frequency (0.0 – 0.1 Hz) band to that in the high-frequency (0.1 – 0.4 Hz) band.8 Elevated low-frequency coupling (e-LFC) is a subset of LFC that, in patients with sleep apnea, correlates with scored apneas and hypopneas. A narrow spectral band in the region of LFC indicates a dominant chemoreflex influence—CSA, periodic breathing, or complex SAS; broad spectral bands specify dominant OSA. It is the construct of e-LFC in the spectrogram that allows the sleep technician or clinician to easily differentiate between obstructive and nonobstructive sleep-disordered breathing.
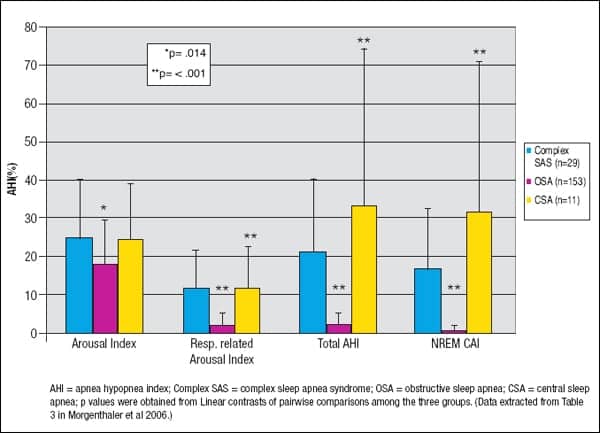 |
| Figure 2. Apnea Hypopnea Index, Respiratory-related Arousal Index, Total Apnea Hypopnea Index, and NREM Central Apnea Index comparison between complex SAS, OSA, and CSA patients on CPAP. |
An e-LFC was found to be superior to nocturnal oxygen desaturation, sleep apnea severity, or the AASM-recommended CAI threshold of Ž5 as the strongest predictor of PAP success or failure.8 In predicting successful PAP titration, e-LFC showed a specificity of 95.2%, sensitivity of 85.7%, positive predictive value of 88.9%, and negative predictive value of 93.7%, and correctly classified 90.9% of the subjects.8 These results suggest that CPC analysis may be an optimal tool to phenotype SDB and identify patients for whom PAP will be successful therapy (or not). CPC analysis will provide sleep technicians and clinicians the opportunity to initiate appropriate successful therapy solutions, reduce probable failure PAP rate, increase PAP adherence, and enhance good medical practice in sleep disorders medicine.
THERAPEUTIC APPROACHES
Typically, CPAP titration starts at 4 to 5 cm H2O pressure and is increased by increments of 1 to 2 cm H2O until obstructive events and snoring are eliminated. On the PSG, persistent arousals resulting from central events and periodic breathing are continued to be titrated in the hope of elimination with higher CPAP, often as high as 5 cm H2O above the pressure that eliminated obstructive events.1 This “over titration” is unsuccessful in eliminating arousals and can result in increased patient discomfort, intolerance of the therapy, and technician frustration.
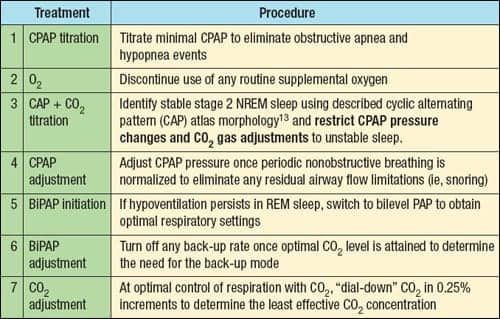 |
Thomas et al titrated CO2 using a novel device, the Positive Airway Pressure Gas Modulator, in six patients with complex SAS using PAP and reported that CO2 levels of 0.5% to 1.5% delivered in increments of 0.25% were highly effective in normalizing respiration.10 The investigators developed an experimental therapeutic protocol for treating complex SAS patients with CO2 following confirmation of SDB (see Table 3).
The supplemental CO2 therapeutic approach in complex SAS is supported by the entire elimination of apneas and Cheyne-Stokes respiration with the addition of a constant FICO2 of 0.03% via full face mask to congestive heart failure patients (n = 6).11,12 Use of dead space with positive airway pressure may also be effective (Thomas, personal communication), but prospective trials of such approaches are required before widespread adoption occurs in clinical practice.
SUMMARY
Upon clinical evaluation, complex SAS patients presented similarly to OSA and CSA patients except for a higher proportion of males and less sleep maintenance insomnia among the complex SAS group. Clinical features do not reliably predict who will display the complex SAS phenotype during positive pressure titration. There is published evidence that counting of standard sleep stages is not sensitive to existent pathology in some sleep disorders8,13-16 and that the indexes derived (ie, AHI) can distort some sleep variables.8,17 Until more refined methods like time-series analysis (ie, CPC analysis) are developed for clinical application, predictive identification of the complex SAS phenotype will be unlikely revealed from standard PSG data.
Education of our patients is as important as that of our health care providers. A friend of mine suggested that if we told our SDB patients that apnea, if untreated, would cause cancer, he thought they would be more motivated to lose weight, work with their CPAP, and stay off their backs while sleeping—in short, they would try harder. I am not one who goes for scare tactics, but all too often I explain the consequences of untreated OSA to patients who tell me that no one has told them what their condition is, what its consequences are, and, most importantly, what they need to do to fix it. When patients understand their condition and its consequences, they are much more likely to do what is necessary to get better.
Mixed apneas composed of a central component followed by an obstructive component are seen in many patients.1,7,17 Complex disease, which presents clinically with mixed apneas, is described in two separate forms, a hypercapnic and hypocapnic type. It is in sleep where the hypocapnic type becomes evident and may in some cases be clearly seen after CPAP initiation with the emergence of periodic breathing and persistent sleep fragmentation. Treatment recommendations, at this point, are for the use of adaptive ventilation,18 PAP with an increase of CO2 as adjunct therapy in the form of a nonvented mask, increasing dead space by adding an extra length of tubing between the CPAP and face mask, or addition of supplemental CO2 in 0.25% increments (when such a device is commercially available). The use of pharmacotherapy in combination with PAP and CO2 is an alternative to those with ensuing insomnia.
As research analytical tools (ie, CPC analysis) become validated and move into the mainstream sleep lab, clinicians and technicians will have additional methods at hand to aid in the differentiation, identification, treatment, and assessment of compliance for complex SAS patients on therapy.
Preetam Schramm, PhD, RPSGT, is principal clinical specialist at Embla Inc, Denver. The author acknowledges the contribution of Dr Robert Thomas at Beth Israel Deaconess Medical Center for his valuable comments, editing, and suggestions on this manuscript. The author can be reached at [email protected].
REFERENCES
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29:1203-1209.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667-89.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Chicago: American Academy of Sleep Medicine; 2001:52-58.
- Thomas RJ, Mietus JE, Peng CK, et al. Differentiating pure obstructive and complex sleep apnea syndrome: utility of an automated electrocardiogram-based method. Submitted to Sleep.
- Rechtschaffen A, Kales A, eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute, UCLA; 1968.
- Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex-sleep disordered breathing. Curr Opin Med. 2005;11:485-493.
- Teschler H, Döhring J, Wang Y, Berthon-Jones M. Adaptive pressure support servo-ventilation, a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614-619.
- Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151-61.
- Morgenthaler T, Gay P, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468-475.
- Thomas RJ, Daly RW, Weiss JW. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep. 2005;28:69-77.
- Ryan CM, Bradley TD. Periodicity of obstructive sleep apnea in patients with and without heart failure. Chest. 2005;127:536-542.
- Steens RD, Millar TW, Su X, et al. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep. 1994;17:61-68.
- Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Regional scalp EEG slow-wave synchronization during sleep cyclic alternating pattern A1 subtypes. Neurosci Lett. 2006;404:352-7.
- Parrino L, Smerieri A, Boselli M, Spaggiari MC, Terzano MG. Sleep reactivity during acute nasal CPAP in obstructive sleep apnea syndrome. Neurology. 2000;25;54:1633-40.
- Scharf MB, McDannold MD, Zaretsky NT, Hux GT, Brannen DE, Berkowitz DV. Cyclic alternating pattern sequences in non-apneic snorers with and without nasal dilation. Ear Nose Throat J. 1996;75:617-9.
- Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di Giovanni G, Terzano MG. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13:314-23.
- Thomas RJ, Terzano MG, Parrino L, Weiss JW. Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern—a recognizable polysomnographic variant with practical clinical implications. Sleep. 2004;27:229-34.
- Cherniack N. Sleep apnea and its causes. J Clin Invest. 1984;73:1501-1506.




