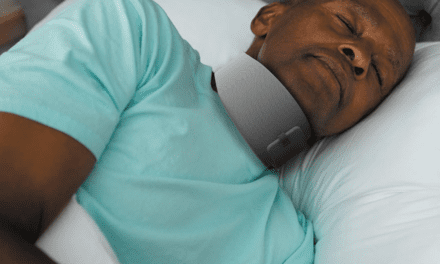
As of the beginning of February, Onera Health has enrolled the first 50 patients into post-market clinical evaluations of its patch-based polysomnography (PSG) solution, with continuous enrollment of more subjects. The patch is OKed in Europe and pending US Food and Drug Administration evaluation.
The first part of the studies will assess the diagnostic accuracy of Onera STS I in comparison with in-lab polysomnography administered by a sleep tech. The second part will evaluate the usability and reliability of Onera STS I for ambulatory PSG, in which patients apply the patches themselves at their homes.
“We are pleased to see Onera’s solution used on a routine basis in an in-lab setting as well as the comfort of the patient’s home. We are eager to collect further evidence that Onera STS I not only meets the gold-standard set by traditional PSG systems but, through its user-centric and compact design, brings additional benefits for doctors and patients alike. We are excited to contribute to a future where this comprehensive diagnostic solution can be delivered to many patients with sleep disorders, whether in a clinical or non-clinical setting,” says Ruben de Francisco, founder and CEO of Onera, in a release.
[RELATED: The Future of Wireless Home Sleep Testing is Here]
Hartmut Schneider, MD, PhD, retired associate professor of pulmonary and critical care medicine at John Hopkins University and Onera co-founder, says in a release, “Technological innovations like Onera’s patch-based PSG system are essential to helping end the delays in identifying various sleep disorders. I’m delighted to see such a seamless integration in our day-to-day clinical practice. The signal quality we have seen with Onera STS I meets our requirements for a comprehensive assessment of sleep disorders. Compared to the commonly used technology, the reduction of hook-up time is remarkable; it literally takes minutes! Additionally, the patients voice positive feedback, which indicates to us a high patient satisfaction, something that we shouldn’t underestimate when it comes to our field.”
With the ongoing enrollment of more subjects in different centers in Europe, the studies are scheduled to complete enrollment in the second quarter of 2022. Clinical study results will be presented in leading medical conferences later this year.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 960826.