Today, the US Food and Drug Administration (FDA) announced a Philips Respironics recall of certain BiPAP and CPAP masks due to a serious safety concern. This is for separate safety concerns from the two recent Philips Respironics CPAP devices recalls.
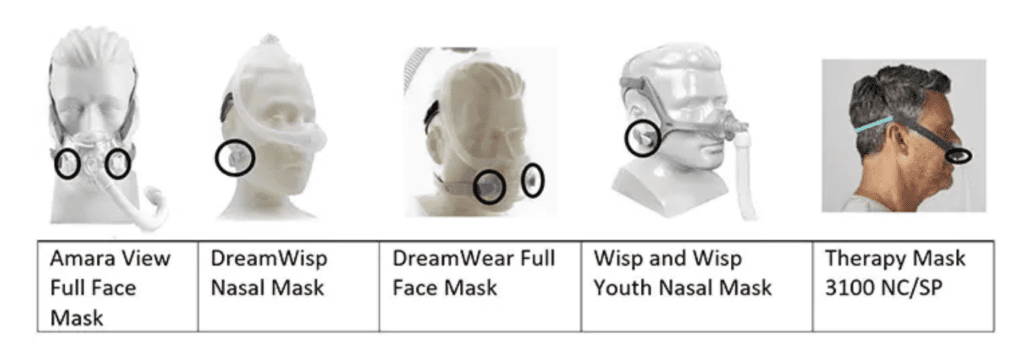
The recalled masks have magnets (placements shown by black circles in the pictures) and can cause potential injuries or death when use of a recalled mask with magnets interferes with certain implanted metallic medical devices and metallic objects in the body.
Medical devices that could potentially be affected by these magnets include brain stents, aneurysm clips, pacemakers, implantable cardioverter defibrillators, ventriculoperitoneal shunts, ocular implants, magnetic denture attachments, insulin pumps, certain neurostimulators used in and around the neck, cochlear implants, or any metallic implanted medical device affected by magnets.
The magnets could also affect mask users who have metallic objects in their body, such as shrapnel or splinters in their eyes, including people near the patient wearing the affected mask, such as a bed partner.
Five mask types are affected by this recall:
- Philips Respironics DreamWisp Nasal Mask;
- Philips Respironics DreamWear Full Face Mask;
- Philips Respironics Amara View Full Face Mask;
- Philips Respironics Wisp and Wisp Youth Nasal Mask;
- and Philips Respironics Therapy Mask 3100 NC/SP.
To date, Philips reported 14 serious injuries, including pacemaker failure, arrhythmia, seizures, and irregular blood pressure related to use of the recalled masks. The FDA is providing recommendations in a safety communication for patients, caregivers, and health care providers concerning use of the recalled masks with magnets, which patients may be using with Philips BiPAP and CPAP machines or those of other manufacturers.
“This latest recall raises further safety concerns both for Philips devices already subject to a recall, as well as additional devices,” says Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We strongly encourage providers and at-risk patients to review this important safety information and follow our recommended actions to reduce the potential for harm from these products.”
“The magnetic headgear clips are used to attach the headgear straps to the masks, which is a method that is commonly used in the sleep therapy devices industry,” states a release issued by Philips Respironics. “This is a voluntary notification to users of specific CPAP or Bi-Level PAP therapy masks containing such magnetic clips to inform them of the updated instructions and labeling. All users should read and follow Philips Respironics’ voluntarily updated warning and added contraindication described below. This represents a new and industry-leading practice.”
Magnets with a magnetic field strength of 400 mT are used in the masks. With the exception of the devices identified in the contraindication, ensure that the mask is kept at least 6 inches (approx. 15 cm) away from any other medical implants or medical devices that can be impacted by the magnetic fields to avoid possible effects from localized magnetic fields. This includes household members, caregivers, and bed partners that may be in close vicinity to patients that use the masks.
In its FAQ, Philips states that, following the implementation of the updated instructions and labeling, Philips Respironics will continue to distribute masks with magnetic clips.
Patients with questions may contact Philips Respironics’ customer service at 1-800-345-6443, (Monday – Friday; 8:30 AM ET to 8:00 PM ET) for more information about non-magnetic mask options. Patients may also contact their durable medical equipment (DME) provider, which supplied the masks affected by this notice.
Any adverse events experienced with the use of masks containing magnetic clips should be reported to the FDA’s MedWatch Program by phone at 1-800-FDA-1088, by fax at 1-800-FDA-0178, by mail at MedWatch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787, or on the MedWatch Web site at www.fda.gov/medwatch.
According to the FDA, more than 17 million masks are impacted by the recall announced by the FDA today. This recall affects masks used with some of the devices that were recalled in June 2021.
Dreamstime ID 98621222 © Oleschwander | Dreamstime.com






Please keep me posted on new development