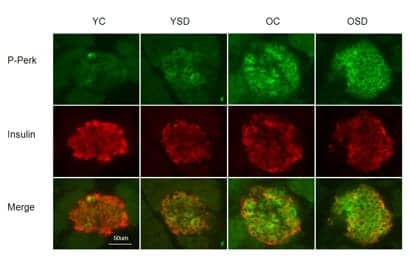
There is increased endoplasmic reticulum stress in the pancreas of aged mice. Phosphorylation of PERK, indicative of endoplasmic reticulum stress and activation of the unfolded protein response, is evident in beta-cells. Representative images of P-PERK (green) in B-cells (red) immunostained for insulin in young undisturbed (YC), young sleep deprived (YSD), old undisturbed (OC) and old sleep deprived (OSD) mice. (Photo by: Nirinjini Naidoo, PhD, Division of Sleep Medicine, Perelman School of Medicine; Aging Cell)
Stress in pancreatic cells due to sleep deprivation may contribute to the loss or dysfunction of the cells important to maintaining proper blood sugar levels, according to a study by Perelman School of Medicine, University of Pennsylvania, published in Aging Cell this month. These functions may be exacerbated by normal aging.
“The combined effect of aging and sleep deprivation resulted in a loss of control of blood sugar reminiscent of pre-diabetes in mice,” says Nirinjini Naidoo, PhD, research associate professor in the Division of Sleep Medicine, in a release. “We hypothesize that older humans might be especially susceptible to the effects of sleep deprivation on the disruption of glucose homeostasis via cell stress.”
Working with Penn colleague Joe Baur, PhD, assistant professor of Physiology, Naidoo started a collaboration to look at the relationship of sleep deprivation, the unfolded protein response (UPR), and metabolic response with age. Other researchers had suggested that the death of beta cells associated with type 2 diabetes may be due to stress in a cell compartment called the endoplasmic reticulum (ER). The UPR is one part of the quality control system in the ER, where some proteins are made.
Knowing this, Naidoo and Baur asked if sleep deprivation causes ER stress in the pancreas, via an increase in protein misfolding, and, in turn, how this relates to aging.
The team examined tissues in mice for cellular stress following acute sleep deprivation, and they also looked for cellular stress in aging mice. Their results show that both age and sleep deprivation combine to induce cellular stress in the pancreas.
Older mice fared markedly worse when subjected to sleep deprivation. Pancreas tissue from older mice or from young animals subjected to sleep deprivation exhibited signs of protein misfolding, yet both were able to maintain insulin secretion and control blood sugar levels. Pancreas tissue from acutely sleep-deprived aged animals exhibited a marked increase in CHOP, a protein associated with cell death, suggesting a maladaptive response to cellular stress with age that was amplified by sleep deprivation.
Acute sleep deprivation caused increased plasma glucose levels in both young and old animals. However, this change was not overtly related to stress in beta cells, since plasma insulin levels were not lower following acute lack of sleep.
Accordingly, young animals subjected to acute sleep deprivation remained tolerant to a glucose challenge. In a chronic sleep deprivation experiment, young mice were sensitized to insulin and had improved control of their blood sugar, whereas aged animals became hyperglycemic and failed to maintain appropriate plasma insulin concentrations.