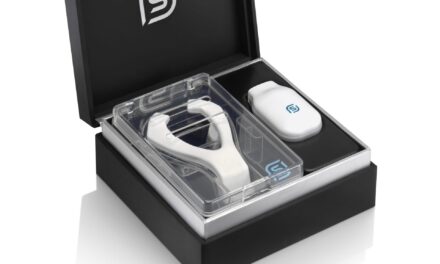
The first generation of Nexalin Technology Inc’s neurostimulator cleared the US Food and Drug Administration (FDA) for the treatment of insomnia in 2003. Nexalin no longer manufactures or sells the gen-1 device in the United States, but it is closing in on an FDA presentation for its next-generation devices for insomnia.
“Our goal has been and continues to be establishing the company as the preeminent producer of innovative neurostimulation devices to uniquely and effectively help combat the ongoing global mental health epidemic,” says Mark White, Nexalin CEO, in a business update and letter to shareholders this month. “With the evolution of our business and regulatory strategy, we have implemented important technology enhancements to our Gen-1 device and waveform.”
The enhancements will increase neurostimulator waveform power from 4 milliamps to 15 milliamps, which the company states will result in a stronger and more powerful waveform that will allow deeper penetration in the brain and provide enhanced patient response without any adverse side effects.

An improved modern enclosure, referred to as generation 2 (gen-2), will administer the new waveform in a clinical setting.
The Nexalin design team has completed prototype designs for the new patient headset, generation 3 (gen-3).
Nexalin is planning to present gen-2 and gen-3 to the FDA in Q1 2023 for the treatment of anxiety and insomnia. Strategic planning for a premarket approval meeting with the FDA for the treatment of major depressive disorder is planned for Q3 2023. A large blinded clinical trial with the gen-3 headset will support the strategy.
Meanwhile, Nexalin gen-2 has been approved for insomnia by China’s National Medical Products Administration. “We are making progress with the commercial rollout of our device in China through our joint venture partner, Wider Come Limited,” White says. “We are currently making strategic plans for additional clinical trials for the use of Gen-2 and Gen-3 devices in China to scientifically validate the new advanced waveform.”
In September 2022, the company was listed on the Nasdaq Stock Market and concurrently raised $9.6 million. “This financing puts us on solid footing to execute our strategy and we believe our listing on a major US exchange provides us increased visibility, liquidity, and the opportunity to attract a broader investor base,” White says. “As a result, we believe we are well funded to support the clinical development, sales, and marketing activities around our innovative medical devices….We remain encouraged by the potential of our neurostimulation devices for improving healthcare outcomes among patients with mental and neurodegenerative illnesses as a proven alternative to psychiatric drugs. We look forward to providing further updates on our clinical research, and commercial programs as we continue to make progress heading into the new year.”