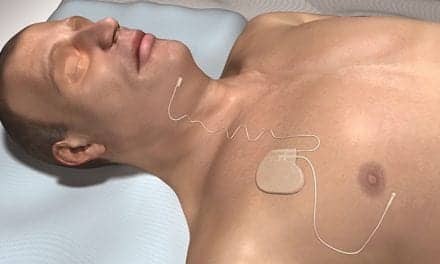
Nyxoah SA announced that the BETTER SLEEP trial reached its primary safety and performance endpoints.
The BETTER SLEEP trial was designed to assess the long-term safety and performance of the Genio bilateral hypoglossal nerve stimulation system in 42 adult obstructive sleep apnea (OSA) patients with and without complete concentric collapse (CCC) of the soft palate. The BETTER SLEEP trial includes a subgroup of CCC patients who are currently contraindicated for unilateral hypoglossal nerve stimulation. The trial was authorized by the Australian and New Zealand regulatory authorities and is being conducted in nine local medical centers
Top-line results from the BETTER SLEEP study showed:
- A statistically significant mean reduction in apnea hypopnea index from baseline to six months post implantation in the whole cohort (CCC and non-CCC patients);
- A statistically significant mean reduction in AHI from baseline to six months post implantation in the complete concentric collapse patient subgroup;
- 42.9% of the study population had complete concentric collapse;
- The company expects to announce additional data with respect to the study as further analyses are conducted.
Richard Lewis, MBBS, FRACS, principal investigator of the BETTER SLEEP study, from Royal Perth Hospital and the University of Western Australia, says in a release, “The top-line results of the BETTER SLEEP study are extremely encouraging. The most impressive aspect of the results is the responder rate of the CCC patient subgroup. These patients are excluded from unilateral hypoglossal nerve stimulation, but the BETTER SLEEP results showed a significant reduction in AHI in these patients following treatment with the Genio system, which is the first device designed to deliver bilateral stimulation of the hypoglossal nerve. Overall, we have achieved a very high responder rate in both CCC and non-CCC patients, and the treatment with the Genio system was well tolerated. We look forward to publishing this data in a leading medical journal.”
Olivier Taelman, CEO of Nyxoah, says, in a release, “We are excited by the top-line data, with the primary performance and safety endpoints met, supporting our belief that the Genio system’s bilateral stimulation of the hypoglossal nerve, has the potential to provide positive clinical outcomes for patients with CCC. We already engaged with the EU Notified Body to review these study data, with the goal of expanding the CE-marked indication to include CCC patients. In parallel, we plan to initiate dialogue with the FDA to further discuss the Genio system as a potential treatment option for patients with CCC. Additionally, we observed a 70% responder rate in the non-CCC patient subgroup based on the Sher criteria, which strengthens our confidence in ongoing study outcomes.”