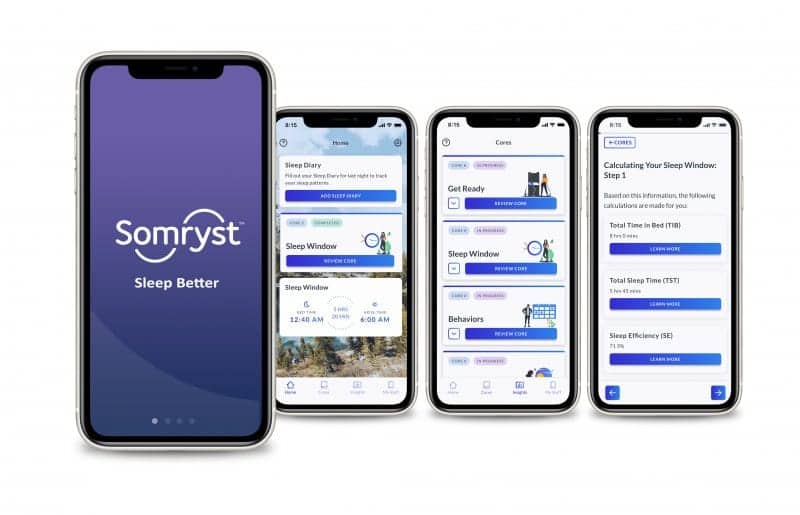
Nox Health’s recent acquisition of Somryst, the first FDA-cleared prescription digital therapeutic for adults with chronic insomnia, deepens the company’s value-based healthcare programs as it seeks to reframe sleep as a tool for managing chronic health conditions.
The company called the acquisition “a major milestone” in its growth journey that will further scale its success.
“The acquisition of Somryst aligns seamlessly with Nox’s mission of promoting science-based solutions that address chronic diseases through sleep care management. As an outcomes-driven company with 10 years of real-world data, it was important for us to find a solution with equal depth in validation and outcomes data, thereby setting it apart from other CBT-I (cognitive behavioral therapy for insomnia) solutions,” says Sigurjon Kristjansson, CEO of Nox Health, in a release.
CBT-I is recognized as a first-line treatment for chronic insomnia, a condition associated with significant adverse health outcomes, by the American Academy of Sleep Medicine and the American College of Physicians. By delivering CBT-I via a mobile application, Somryst offers health care providers a novel treatment delivery modality to enhance the treatment of chronic insomnia for patients who lack access to clinician-delivered CBT-I, according to a release from Nox Health.
Pivotal study results show that over 40% of Somryst-treated patients no longer met criteria for chronic insomnia post-treatment, and over 60% demonstrated a clinically meaningful insomnia treatment response with no adverse events reported.
Nox Health now aims to bring Somryst to a wider audience, starting with its existing Enterprise client base and expanding to other opportunities throughout its business.
The company agreed to pay $3.9 million to acquire Somryst from Pear Therapeutics, which filed for Chapter 11 bankruptcy last month. An auction to divest the company’s assets was held last week.