|
Can LTF provide a treatment option for OSA?
By Regina Patrick, RPSGT
Long-term facilitation (LTF) is an increase in nerve activity that persists minutes to hours after the cessation of a stimulus. For example, multiple, brief episodes of hypoxia can induce LTF in respiratory motor nerves such as the phrenic and hypoglossal nerves. LTF of the phrenic nerve results in increased depth and rate of inspiration, and LTF of the hypoglossal nerve results in depression of the base of the tongue with inspirations. Scientists are not sure why hypoxia induces LTF in the respiratory nerves. One possibility may be that the increased depth of inspiration and depression of the tongue base induced by LTF during hypoxia open the upper airway so that more air can flow into the respiratory tract. With this in mind, researchers hope that some day LTF could be used to treat obstructive sleep apnea (OSA).
The discovery of respiratory LTF is an outgrowth of research investigating the rhythmicity of respiration. By the turn of the 20th century, scientists had concluded that oxygen and carbon dioxide were the stimuli that drove the rhythmicity of respiration. In 1911, Hans Winterstein1,2 proposed that O2 and CO2 brought about respiratory rhythmicity by acting on a single chemoreceptor. In 1956, he revised this proposal and suggested instead that two types of receptors existed: a central chemoreceptor and a peripheral chemoreceptor. Scientists later validated this proposal on learning that the aortic bodies and the carotid bodies are the peripheral chemoreceptors involved in respiratory rhythmicity. The aortic bodies are located on the arch of aorta, and the carotid bodies are located within the fork of the carotid arteries in the neck. Animal studies3,4 demonstrate that interrupting neural input from the aortic or carotid bodies can reduce ventilatory responses to hypoxia or hypercapnia.
RESPIRATORY CHEMORECEPTORS
Finding the location of the central respiratory chemoreceptors proved more elusive. In the 1960s, scientists were focusing on a certain area of the upper medulla—the ventrolateral medulla (VLM)—as the brain region containing the central respiratory chemoreceptors. For example, in 1963 University of California researcher Robert Mitchell and colleagues5 in their animal study noted no change in the breathing rate when they increased the acidity of the cerebrospinal fluid (CSF) in the brain’s fourth ventricle, a fluid-filled cavity that forms a narrow passage through the pons, the medulla, and the cerebellum. A thin layer of tissue (the meninges) forms a barrier between the fourth ventricle and the surface of the medulla. Since this may have prevented the expected increase in breathing (other researchers had found that increasing the acidity of the CSF in the brain’s ventricles increased the breathing rate2), Mitchell and colleagues directly bathed the surface of the ventrolateral medulla with an acid solution. This time there was an increase in the breathing rate. From this, they concluded that the chemoreceptors controlling the respiration rate were located on the surface of the ventrolateral medulla.
|
Subscribe to Sleep Report to receive OSA research updates |
Scientists now are aware of the existence of several nuclei in the pons and medulla that control various aspects of respiration. Collectively, these nuclei are called the “respiratory center.”
CENTRAL AND PERIPHERAL CHEMORECEPTORS
Researchers continue to try to understand the neural interactions between the central and peripheral chemoreceptors. To this end, many studies have investigated the impact of artificially stimulating central and peripheral respiratory chemoreceptors or the impact of disrupting their neural input. Through these techniques, Millhorn and colleagues6 in 1980 discovered the phenomenon that is now called ventilatory long-term facilitation. In their study on cats, they noted an increased inspiratory response when electrically stimulating either the peripheral or the central respiratory chemoreceptors. The animal’s inspiratory activity returned to its prestimulation level within minutes when they stopped stimulating central chemoreceptors. In contrast, when they stopped stimulating the peripheral chemoreceptors, the inspiratory activity remained elevated for up to 90 minutes. This prolonged increase in respiration following peripheral chemoreceptor stimulation occurred even if the animal had undergone vagotomy; had been surgically paralyzed by cutting the upper spinal cord; or had undergone decerebration (a procedure that interrupts cerebral activity from being transmitted through the rest of the brain). These procedures normally block or weaken respiratory reflexes that are modulated by peripheral chemoreceptors. The persistent activation in respiration despite these procedures indicated the activation of a central mechanism. Millhorn and colleagues concluded that they had discovered the presence of a new brainstem neural mechanism that is activated by peripheral receptors, but, once activated, maintains respiration at an increased rate for a long period of time.
The term “long-term facilitation” was first used in 1993 by Hayashi and colleagues7 to describe the phenomenon that Millhorn had noted. They recorded phrenic nerve activity in rats during and after three episodes of hypoxia. Each hypoxic episode lasted 5 minutes and was separated by 10 minutes of hyperoxia. They noted that the burst frequency of the phrenic nerve and the amplitude of the nerve’s activity remained higher than its prehypoxic level at 30 minutes and 60 minutes after the last hypoxic episode.
LTF AND OSA
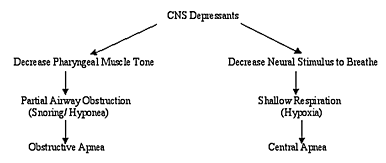
Many studies on LTF have focused on the phrenic nerve. This nerve innervates the diaphragm. The diaphragm contracts when the nerve is activated, resulting in an inspiration. The more activated the nerve, the deeper and faster is one’s respiration. However, respiratory LTF is not limited to the phrenic nerve. It also can occur in the hypoglossal nerve (ie, cranial nerve XII), which innervates the genioglossus muscle and the hypoglossus muscle.8 Normally, activation of the hypoglossal nerve causes the genioglossal and hypoglossal muscles to depress the base of the tongue during inspirations. This action opens the upper airway, allowing air to quickly enter the respiratory tract. Phrenic nerve LTF in combination with hypoglossal nerve LTF may prevent the occurrence of sleep apnea in people with upper airway restriction or mild sleep apnea.
In support of this, scientists have noted that LTF of the upper airway muscles occurs in people who have upper airway restriction, but it does not occur in nonsnorers and it does not occur in people whose sleep apnea is relieved by continuous positive airway pressure treatment.9,10 Also, about 80% of people diagnosed with OSA do have long periods of stable breathing during sleep; the remaining 20% of OSA patients who do not have long periods of stable breathing during sleep also do not have LTF of the upper airway muscles.11
Scientists are not fully clear how LTF occurs. One proposal looks at the interaction between serotonin, nerve growth factors, and cellular signaling molecules.11,12
In this proposal, an episode of hypoxia causes serotonergic neurons in the brainstem to release the neurotransmitter serotonin. Serotonin travels across the synapse to bind with receptors on the motoneurons in a motor nerve’s nucleus (eg, phrenic motor nucleus) in the brainstem. This activates the synthesis of the protein brain-derived neurotrophic factor (BDNF) within the motoneurons. The motoneurons then release BDNF. Once released, some of the BDNF binds to and activates tyrosine kinase receptors located on the surface of the motoneuron. This activation may trigger the production of other kinase enzymes within the motoneuron. These kinase enzymes may then enhance and prolong the neurostimulatory actions of glutamate, thereby inducing LTF.
A second pathway11 that may contribute to LTF involves the activation of G protein-coupled adenosine receptors on the surface of the motoneuron. Activation of these receptors may trigger the synthesis of various kinases including tyrosine kinase. The kinases may in turn enhance the stimulatory actions of glutamate on the motoneuron and/or result in the creation of new NMDA and AMPA receptors. NMDA (N-methyl-D-aspartate) is an excitatory neurotransmitter similar to glutamate. When glutamate binds to an NMDA receptor on the surface of a motoneuron, the cell becomes activated. AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) is a glutamate agonist. That is, it can bind to the glutamate receptor in place of glutamate yet induce the same stimulatory response in a neuron. Therefore, increased numbers of NMDA and AMPA receptors on the surface of a motoneuron may strengthen excitation in the motoneuron.
USING LTF TO TREAT OSA
Potential treatment options using LTF to treat OSA could involve either the strategic use of hypoxia, such as using hypoxia for a short period of time during the daytime to elicit the effects of LTF during the night; gene therapy aimed at regulating BDNF synthesis; drug therapy aimed at inhibiting the actions of proteins that otherwise would inhibit LTF; or the use of agonist drugs that induce LTF. These treatment options have not yet been fully investigated. Recently, however, a rat study11 successfully used gene therapy to modulate BDNF production in the rat phrenic motor nucleus. The researchers of the study cautioned that targeting the desired genes and proteins in humans may be more problematic.
The most successful therapy for OSA is continuous (or bilevel) positive airway pressure (CPAP or BPAP, respectively) treatment. Because of physical discomfort or other factors such as the bulkiness of the machine, only about 40% of OSA patients comply with CPAP or BPAP treatment consistently.11 Surgical treatment for OSA such as uvulopalatopharyngoplasty (UPPP) often can not abolish episodes of apneas completely. Pharmacological approaches for OSA treatment have had inconsistent or disappointing results in various research studies. For example, researchers have found that selective serotonin reuptake inhibitor (SSRI) drugs can reduce apneas in non-rapid eye movement (NREM) sleep but not in REM sleep.11 Therefore, there is currently no drug therapy for OSA. These drawbacks of CPAP/BPAP therapy, surgery, and drug treatment make it necessary to investigate new treatment approaches for OSA. LTF may offer new treatment approaches.
Regina Patrick, RPSGT, is a contributing writer for Sleep Review.
REFERENCES
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1-24.
- Remmers JE. A century of control of breathing. Am J Respir Crit Care Med. 2005;172:6-11.
- Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91(1):328-335.
- Gonzalez F Jr, Fordyce WE, Grodins FS. Mechanism of respiratory responses to intravenous NaHCO3, HCl, and KCN. J Appl Physiol. 1977;43(6):1075-1079.
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963;18:523-533.
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41(1):87-103.
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve response to carotid afferent activation: intact vs decerebellate rats. Am J Physiol. 1993;265:R811-R819.
- Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol. 2005;98:1761-1767.
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21(7):709-716.
- Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol. 2003;94(1):53-59.
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnea? Exp Physiol. 2007;92:27-37.
- Mitchell GS, Baker TL, Nanda SA, et al. Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466-2475.