Insights from disposable home sleep test experts at Sunrise, SleepImage, and ZOLL Itamar about technological advancements, new features, and other aspects of the present and future of sleep testing.
By Lindsey Nolen
Sleep Review recently conducted a roundtable to delve into the status of disposable home sleep testing (HST). The discussion brought together leading voices in the field, offering insights into the latest technological advancements, features, integrations, and challenges.
The panelists were:
- Laurent Martinot, co-founder and CEO, Sunrise

- Bogi Palsson, CEO, SleepImage

- Melih Alvo, senior director, commercial marketing, ZOLL Itamar

- (ResMed was unable to speak with us by our deadline).
Here are their insights.
Pivotal Technological Advancements
From innovation in biosignals and data processing to sensor technology and connectivity, the panelists share their perspectives on the technological advancements that reshaped the landscape of sleep apnea diagnostics.
Martinot: The study and emergence of new biosignals—like mandibular jaw movements, peripheral arterial tone, and tracheal sound analysis—have increased the amount of information that can be gathered with simple, easy-to-use devices.
Advances in data processing capabilities and the application of machine learning algorithms have enabled a more sophisticated analysis of these biosignals.
The miniaturization of sensors, such as accelerometers and gyroscopes, has made it possible to capture essential data in compact, portable devices. It allows patients to apply sensors themselves and maintain a low failure rate without compromising data quality.
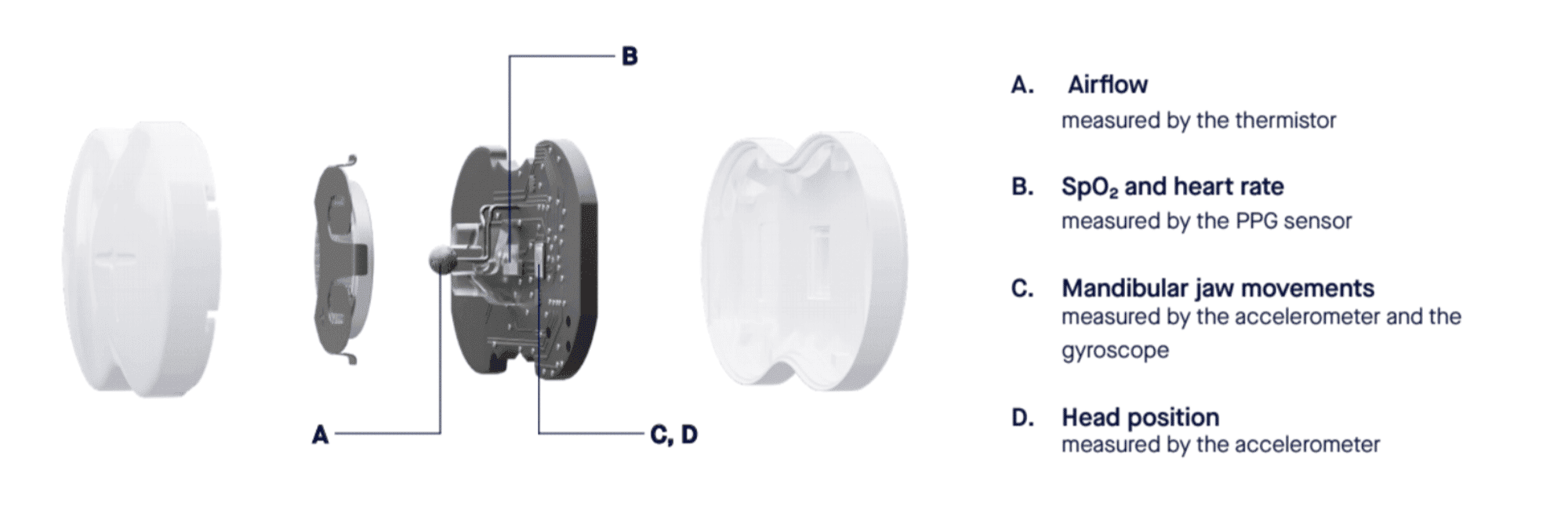
The integration of wireless connectivity features has streamlined data transmission from the home device to healthcare providers.
Palsson: Simplicity of design and ease of use using Bluetooth connectivity between the recording device and the patient’s mobile device. This can be used as a relay to temporarily store and transfer the data at the end of the test and has helped with viability for the patient.
Reduction or elimination of multiple different sensors has helped with financial viability.
Validated advancements in sensor technologies, such as the photoplethysmography (PPG) sensor, have helped with the technical feasibility of gathering high-fidelity physiological data that embeds multiple channels for analysis.
Regulatory pathways such as Software as a Medical Device using cloud-based systems for analysis, reporting, and storage of the physiological data and the generated output have helped with clinical viability.
Disposable HST Options
The panelists discuss their companies’ approaches to HST, highlighting the features, sustainability efforts, competitive advantages, and integrated patient engagement strategies that distinguish their products.
Martinot: Sunrise takes a pioneering approach by utilizing mandibular jaw movement biosignals in conjunction with automated machine learning analysis. This combination revolutionizes the understanding of sleep fragmentation and respiratory effort, thus shedding light on the obstructive or central nature of respiratory events during sleep.
Since day one, Sunrise has fostered close collaboration with a network of distinguished key opinion leaders. These experts bring their wealth of knowledge and experience to the table, significantly enhancing the credibility and effectiveness of our solution.
Sunrise is committed to sustainability. We offer comprehensive return programs for our devices, allowing them to be repurposed. This eco-conscious approach not only benefits the environment but also reflects our dedication to responsible healthcare practices.

Palsson: The biggest differentiator is that the device Sleeplmage offers is at a competitive price point compared to disposable devices without being disposable. We call this a “personalized” device as it can be used by the patient in a similar manner to how a blood pressure monitor can be used over time to track treatment efficacy if the patient is diagnosed with sleep apnea.
The reported output parameters are the same from all SleepImage tests regardless of whether the recording device is intended for use by a single patient or multiple patients. The benefit of the personalized device is it can be used as long as needed or desired during treatment (where the patient is diagnosed with sleep-disordered breathing), making it possible for clinicians to offer their patients improved precision management of their condition with output metrics that reflect the efficacy of treating the apnea part of sleep—but also to manage sleep quality over time, which often gets compromised on treatment if not titrated correctly.

Alvo: WatchPAT ONE calculates the apnea-hypopnea index based on true sleep time, provides sleep staging, and distinguishes central sleep apnea from other types of sleep apnea.
WatchPAT ONE’s latest feature identifies atrial fibrillation and premature beats during the sleep study.
Together with the SleePATh patient mobile app, which integrates subjective patient data with the WatchPAT home sleep study results, all WatchPAT devices allow providers to send screening questionnaires before the sleep study. SleePATh not only helps expedite the preauthorization process but also enhances diagnosis with pre- and post-study questionnaires.

Accuracy and Reliability
The panelists offered insights into how their respective devices function to accurately and reliably assess sleep apnea.
Martinot: Sunrise’s three-night home sleep test uses an 8-gram sensor placed on the chin to record mandibular jaw movements. It accurately detects key mandibular jaw movements and biosignal patterns related to various sleep events, like sleep stages, arousals, and different types of breathing disruptions, such as apneas, hypopneas, and respiratory event-related arousals. These data points are turned into clinical scores and brought together in a comprehensive, automated report.
Clinicians have the option of reviewing the raw data and modifying the report using the hypopnea 1A and 1B rules, based on 3% and 4% oxygen desaturation.
Our accuracy has been rigorously evaluated against in-lab PSG on a sample of 389 patients, and the results have been published in JAMA Network Open. With a PSG cutoff of at least five events per hour, Sunrise demonstrated an accuracy of 0.92 and a post-test probability of 0.99. For a PSG cutoff of at least 15 events per hour, the accuracy was 0.88, with a post-test probability of 0.89. These robust results are supported by positive likelihood ratios of 14.86 and 5.63, respectively.1
Palsson: The SleepImage Sleep Quality Index is an FDA-cleared unit of measure for sleep that has been associated with health outcomes, such as blood pressure control, in peer-reviewed publications. It is thus very important to treat both the apnea and sleep itself for successful outcomes from the treatment of sleep-disordered breathing.
SleepImage has published all the parameters such as sensitivity, specificity, agreement, and other metrics from our FDA clearance in a document called “Introduction to SleepImage.”2 The demonstrated accuracy is what backs up a statement in the FDA clearance letter to state that the SleepImage output is comparable to output from PSG studies at all severity levels of sleep apnea in both children and adults.
Alvo: WatchPAT ONE and our reusable version, WatchPAT 300, use peripheral arterial tonometry technology and six additional channels to record oximetry, pulse rate, actigraphy, body position, snoring, and chest movement for sleep apnea diagnosis. Both devices have the same functionality and offer accurate and reliable home sleep apnea testing.
Updates Since Introduction
The panelists provided updates on the enhancements their devices have undergone since launching, detailing improvements driven by technological advancements and user feedback.
Martinot: Since its initial introduction, our device has undergone significant evolution in response to user feedback and advancements in technology. The journey began with the introduction of a new biosignal to the market, utilizing an accelerometer and gyroscope to capture mandibular jaw movements. This led to the FDA granting de novo approval in 2021.
We further refined the device by incorporating additional channels, including oximetry and airflow. This not only enhanced functionality but also aligned with the prevailing reimbursement framework and facilitated smoother adoption by clinicians. By the end of 2022, the second iteration of our device received FDA clearance.
In mid-2023, the enhanced device was commercially launched. Since then, we have maintained a relentless focus on continuous improvement. This includes ongoing software refinements based on user feedback, with an emphasis on streamlining the clinician workflow. This involves simplifying processes such as re-scoring a study, running 3% or 4% analyses, and introducing account management features.
We have introduced new APIs and integrated them with middleware to ensure seamless compatibility.
Palsson: Our device is primarily the cloud-based Software as a Medical Device, which did not change by the addition of a “disposable” hardware device as that hardware uses the same input signals collected by the same sensor type (PPG) with the same data acquisition characteristics as the hardware that is intended for multi-patient use.
Alvo: Two years ago, we introduced the SleePATh patient mobile app with pre- and post-sleep study questionnaires that are integrated into a WatchPAT sleep study. Features are regularly added to this platform, including screening questionnaires and patient education tools, which help clinicians expedite the diagnosis process and save time for them and their staff.
This year, we also introduced an arrhythmia detection feature in the United States to identify atrial fibrillation and premature beats in all our products. This new feature will help clinicians flag those patients suspected of having an arrhythmia.
Integrating Disposable HST with Other Devices
The panelists shared their perspectives on the integration of HST devices with other health monitoring technologies, discussing the potential benefits and challenges.
Martinot: I envision a future where these technologies seamlessly collaborate, offering clinicians a more holistic understanding of an individual’s health and well-being. This integrated strategy promises to revolutionize health monitoring, ultimately leading to more personalized and effective care.
Wearables are instrumental in the early stages of the healthcare journey. They play a crucial role in patient education about various conditions, aid in early detection, and facilitate smooth communication with healthcare professionals.
Palsson: Sleep-disordered breathing is a chronic medical condition that needs to be managed over time. We believe that personalized devices that can be used repeatedly over time, like blood pressure monitors, are the answer to improved patient care. Disposable devices, on the other hand, are driven by convenience for a point in time use, which comes with challenges, not least the environmental impact that they may have.
It is our belief that every patient who has been diagnosed with sleep apnea should have in their possession a medical device that can accurately track the success of their treatment with clinically validated and meaningful output that reflects health outcomes of conditions that are known to be associated with untreated or poorly treated sleep apnea.
Cardiovascular and cardiometabolic diseases, as well as mental health, top the list of conditions that should be improved by successful treatment of sleep apnea. Disposable devices that may be limited to measuring only the apnea, but not sleep itself, will not provide optimal value or be actionable information.
Challenges
Addressing challenges within the HST market, the discussion highlighted the complexities of designing user-friendly interfaces and the potential for competition in pricing strategies.
Martinot: We’re dedicated to crafting intuitive and user-friendly interfaces for both patients and clinicians without compromising on accuracy and reliability. It’s not always easy; simplicity can be surprisingly tough to achieve in development.
Palsson: There are no company-specific challenges in the disposable home sleep testing market for SleepImage. The opportunity that we see with the ability to compete on price with disposable tests is the ability to offer continuous tracking of treatment efficacy for those who are diagnosed and start treatment.
Alvo: We do not see many challenges as WatchPAT ONE is a very well-accepted device in the sleep community, which provides a comprehensive sleep study in a simple way.
Mitigating Environmental Impacts
The discussion around mitigating environmental impacts focused on the commitment to recycling, providing return programs, and offering long-term reusable devices.
Martinot: Sunrise prioritizes recycling materials over disposal when designing its products to reduce the environmental impact of waste. For instance, Sunrise packaging and accompanying materials are paper-based and fully disposable.
For the sensor, Sunrise offers return programs where the patient can send the device back for proper disposal or recycling. Shipping labels and instructions are provided to the final user. The returned device is disassembled by Sunrise, and recyclable components are separated from non-recyclable ones.
Local recycling guidelines, including e-waste recycling guidelines for electronic components, are followed by Sunrise for proper and safe disposal or recycling.
Palsson: There is no need to dispose of the SleepImage device that competes with disposable devices, so recycling can follow the same path as any other electronic device with rechargeable batteries when it comes to the end of the device’s lifetime. For the SleepImage device, that can be years down the road after the patient may have had the opportunity to use it to help manage treatment through fine-tuning of the therapy device titration and lifestyle changes that may help make sleep apnea treatment more successful.
Alvo: We offer a simple and free program to all WatchPAT ONE patients. Once a patient completes their sleep study with WatchPAT ONE, they can create their free return label to ship the product back to us by visiting our Green Program page.
Potential of Disposable HST
The panelists highlight the role of disposable HST in promoting widespread sleep testing, enhancing patient care, improving affordability and accessibility, and reducing logistical burdens on healthcare providers.
Martinot: We advocate for a paradigm where everyone undergoes a sleep test at least once in their lifetime. While there will always be situations that require an in-lab study, we firmly believe the technology available today allows for reliable at-home sleep testing. This approach not only enhances patient care but also improves affordability and accessibility for clinicians, payers, and patients. Moreover, employing disposable home sleep tests alleviates the logistical burden on healthcare providers, affording them more time to focus on delivering superior patient care.
Palsson: Personalized medical devices open the potential to obtain data points over time that can be used to improve the practice of precision care. It is not enough to treat the apnea, improve compliance metrics, and see health outcomes and quality of life improve. In our view, it is fundamental to treat sleep itself as part of the sleep apnea treatment. That can only be done based on verifiable and objective metrics that reflect health outcomes. When that is done successfully, it is likely that the patient will feel the benefit of the treatment outweighs the inconvenience that many patients feel from sleep apnea treatment.
Alvo: In the US alone, over 54 million adults have sleep apnea, and 80% remain undiagnosed. It is difficult to manage this huge population exclusively with in-lab tests and reusable home sleep apnea tests as clinics are limited by their number of beds and devices. On average, an in-lab sleep test takes a few months to schedule, and a reusable HSAT may take up to seven days to obtain results. This creates patient backlogs. However, single-use devices like WatchPAT ONE can help obviate bed and device limitations, expand the reach of sleep clinics, and help expedite time to diagnosis.
References
- Pépin JL, Letesson C, Le-Dong NN, et al. Assessment of mandibular movement monitoring with machine learning analysis for the diagnosis of obstructive sleep apnea. JAMA Netw Open. 2020 Jan 3;3(1):e1919657.
- Introduction to SleepImage. Available at https://sleepimage.com/wp-content/uploads/Introduction-to-SleepImage.pdf.
Photo 31493254 © Melpomenem | Dreamstime.com









