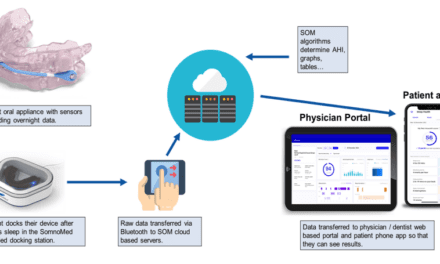
Compare 6 temporary oral appliances (also known as transitional, interim, or trial appliances) side-by-side. Click on the thumbnail or 0619 Temporary Oral Appliances to open the guide at a legible size or print it.
The guide compares features such as FDA status, how it works, time to fit, fitting description, adjustment description, materials, efficacy data, predictive value data, warranty, estimated useful life, payer reimbursement status, and research supporting the appliance for the following devices: Airway Management myTAP, Advanced Brain Monitoring Inc Apnea Guard, Glidewell Laboratories Silent Nite sl, BlueSom BluePro, Healthy Start/Ortho-Tain Snore-Cure, SomnoMed Inc SomnoDent ALPHA.
Also, see our Custom Oral Appliances for Sleep Apnea Comparison Guide.
A version of this comparison guide published in Sleep Review‘s June/July 2019 issue. Information based on data submitted by oral appliance marketers. Sleep Review strives for accuracy but cannot be held responsible for claims made by marketers. All temporary oral appliances may not be included. E-mail sroy[at]medqor.com to be considered for the next update.