From candidacy to implantation to nightly use, the neurostimulator startup process is inherently lengthy. Sleep specialists can explore ways—from practical to novel—to make the process more efficient.
By Alyx Arnett
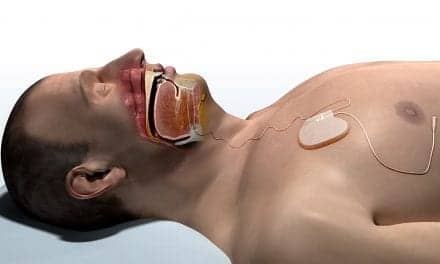
Treating sleep apnea with a neurostimulator, such as Inspire for obstructive sleep apnea (OSA) or remedē for central sleep apnea (CSA), requires much more than one clinic visit. Steps include identifying a candidate, scheduling and undergoing implantation, activating the device, and titrating it—all before finally having the potential for optimal nightly use.
Delays can occur at any stage, prolonging the process and leaving patients untreated. “Patients often have significant symptoms such as fatigue and daytime sleepiness and may drop their oxygen saturations hundreds of times each night,” says Jacob Johnson, senior manager of clinical training and operations at ZOLL Medical, maker of remedē. “Improving these wait times is important for patient care.”
Also, for many patients, the process of getting a neurostimulator follows an already extensive period of sleep apnea diagnosis and trials of other treatments. By the time they begin the neurostimulator startup process, they may feel discouraged.
Holly Behling, marketing associate at Inspire Medical Systems, maker of Inspire therapy, says sleep specialists should set expectations upfront and ensure patients understand the steps and expected timeline.
Sleep specialists can also consider ways—from practical to novel—to move through the neurostimulator process more efficiently.
Efficient Appointments
To prevent early delays, sleep specialists can offer online scheduling, which can help by allowing patients to book appointments directly, avoiding long hold times and delayed callbacks, says Behling.
Additionally, Johnson says, “Creating dedicated clinic time and prioritizing consults for patients who are struggling with mask therapy may reduce the time to effective treatment.”
For patients who require an updated (or initial) sleep test, Behling suggests offering home sleep tests. “That does tend to speed up the process” versus requiring an in-lab polysomnogram (PSG), she says.
Having auto reminders for patients, such as text messages, emails, or calls, can help ensure patients don’t miss their appointments, says Behling.
Help Working With Payors
Third-party payor requirements can add another layer of complexity to starting on neurostimulators. Prior authorization, shifting coverage criteria, and documentation requirements may create obstacles.
For remedē candidates, insurance-related delays are the most common roadblock, according to Johnson. “Documenting the diagnosis of CSA clearly in the chart and indicating why remedē is the next logical step can improve the approval process,” he says.

Via ZOLL, physicians can also request support getting remedē approved from a third-party service called PRIA. PRIA works with healthcare providers and patients, serving as an advocate to facilitate insurance approvals. Once a physician prescribes remedē, PRIA assists in gathering necessary documentation, submitting prior authorization requests to insurers, and managing appeals if coverage is initially denied. “Their expertise is particularly valuable for new and innovative therapies like remedē, where payers may not yet have standardized coverage policies. This service helps reduce the administrative burden on clinics and improves access to therapy for eligible patients,” Johnson says.
For Inspire therapy, prior authorization requirements vary by insurance carrier and frequently change, also making it difficult for providers to know exactly what is required for approval, says Behling. For example, patients may be tasked with losing weight to meet shifting BMI thresholds first.
To minimize disruptions, sleep specialists can proactively review insurance requirements, understanding that they can differ from patient to patient. Having dedicated staff who handle prior authorizations and know how to gather and submit the required documentation efficiently can help move the process along.
Single-Session DISE and Implantation Cut Wait Times
Inspire therapy candidates must undergo a drug-induced sleep endoscopy (DISE) before implantation to rule out complete concentric collapse of the palate.
Typically, DISE is performed separately from implantation, requiring two procedures on different days. But the University of Michigan Health began piloting a pathway in 2021 in which select patients undergo DISE and implantation in the same session.
To qualify, patients underwent a volitional snore test—a procedure where they lie flat and imitate a snoring sound while being evaluated with a scope. This test has been shown to reliably predict which patients would be suitable for confirmatory DISE at the time of implantation.1 If their airway pattern met the criteria, they proceeded to DISE, immediately followed by implantation on the same day.
Nicholas R. Lenze, MD, MPH, of the University of Michigan Medical School, and co-investigators evaluated the outcomes of this pathway.2 Compared to the traditional approach, it reduced wait times by almost four months on average. The study also found it saved hospitals an average of $9,258 per patient. The streamlined pathway was not associated with worse surgical outcomes.
One potential risk, he notes, is holding the operating room time. However, the risk of a patient not qualifying for implantation after DISE appears minimal, as the volitional snore test was highly predictive of DISE findings, with 93% sensitivity and a positive predictive value of 82.9%.
Initially approved by Blue Cross Blue Shield of Michigan, the pathway is now also covered by Medicare at the University of Michigan Health. Lenze believes broader insurance acceptance is feasible due to the demonstrated cost savings.
“The streamlined pathway is one way that hospitals can reduce costs, and it also has the benefit of expediting surgery for these patients who already face delays in care, oftentimes even before the initial diagnosis of obstructive sleep apnea,” says Lenze.
Nighttime In-Lab Titration Alternatives
After implantation, patients typically wait four to six weeks before device activation while they are healing. The devices are then gradually up-titrated at home to find the right therapy level. Inspire Medical then requires an in-lab PSG for titration.
In-lab nighttime PSGs can be logistically challenging, potentially leading to additional delays. To help mitigate these issues, sleep specialists can consider daytime titrations, says Nico de Vries, MD, PhD, an ENT specialist at OLVG West Hospital in Amsterdam, The Netherlands. De Vries co-authored a study evaluating the efficacy of daytime PSG for titration in OSA patients with neurostimulators.3

The study found that although patients slept significantly less during daytime PSG, the session provided enough time to complete titration in 84% of cases. Nearly all (94%) of patients reported a positive experience, and respiratory outcomes improved after titration and remained stable at the 12-month follow-up.
As patients in the study were required to stay awake the night before titration, investigators also looked at how their sleep architecture was influenced. They found no significant effects. Three days after titration, 88.2% of patients did not experience lingering tiredness and could resume normal sleep without issue.
“So there are no downsides,” says de Vries. “We were very happy with it.”
The study also raised another question: Are in-lab titrations necessary? De Vries suggests they may not be for all patients. Device settings changed for 47.4% of patients—meaning that for more than half, optimal settings had already been established during home up-titration.
De Vries proposes that a home sleep test could help determine which patients actually need in-lab titration.“It’s simpler than we thought it was,” he says.
In-lab PSG is not required following activation to titrate the remedē system. “While many providers opt for an overnight sleep study within six months of initiating therapy, this is not required by ZOLL. Some providers rely on remedē diagnostics to evaluate efficacy and guide titration decisions, reserving in-lab studies only when additional insights are necessary,” Johnson says.
Refining Patient Selection
While DISE remains the gold standard for evaluating Inspire neurostimulator candidates, research is exploring ways to enhance its predictive value by incorporating additional evaluation techniques.
One involves assessing airway changes with mandibular advancement during DISE. According to Graeme B. Mulholland, MD, FRCSC, director of sleep surgery and assistant professor in the Division of Otolaryngology – Head & Neck Surgery at the University of Alberta, the assessment may help determine which patients are more likely to benefit from neurostimulation.
In a study of 46 patients, Mulholland and his co-investigator found that those who showed significant airway improvement in the upper pharynx with mandibular advancement during DISE appeared less likely to succeed with neurostimulation.4
Another screening method involves using positive airway pressure (PAP) during DISE. A separate study, in which Mulholland was not involved, of OSA patients undergoing nasal PAP during DISE found those who responded well to neurostimulation had significantly lower palatal opening pressures than non-responders.5 Researchers identified a palatal opening pressure below 8 cm H₂O as a threshold associated with a high predictive value for positive outcomes.
“The idea is from the perspective of further refinement of the selection criteria so that we have a better idea that the people who are getting the implants have a higher chance of success,” Mulholland says.
Although these additional screening methods may introduce an extra layer in the process, they could ultimately prevent patients from enduring long wait times for a treatment that may be less effective for them.
Mulholland emphasizes the importance of strong collaboration between sleep specialists and otolaryngologists to ensure a smoother process for patients. “A good relationship between a sleep center and a surgeon who has the capacity to incorporate a reasonable volume of hypoglossal nerve stimulation into their practice is the key thing,” he says.
References
- Yalamanchi P, Mott N, Ali SA, et al. Evaluation of in-office volitional snore as a screening tool for candidacy for hypoglossal nerve stimulation. Otolaryngol Head Neck Surg. 2022;166(3):595-7.
- Lenze NR, Garcia J, Vijayakumar P, et al. Implementing a streamlined hypoglossal nerve stimulator pathway: cost, time to surgery, and outcomes. OTO Open. 2024 Oct 2;8(4):e70007.
- Bosschieter PFN, Schoustra E, de Vries N, et al. Daytime polysomnography to perform titration for upper airway stimulation in patients with obstructive sleep apnea. Sleep Breath. 2022;26(2):707-15.
- Mulholland GB, Dedhia RC. Success of hypoglossal nerve stimulation using mandibular advancement during sleep endoscopy. Laryngoscope. 2020 Dec;130(12):2917-21.
- Seay EG, Keenan BT, Schwartz AR, et al. Evaluation of therapeutic positive airway pressure as a hypoglossal nerve stimulation predictor in patients with obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2020 Aug 1;146(8):691-8.
Photo caption: A photo illustrates a physician discussing the Inspire neurostimulator for the treatment of obstructive sleep apnea with a prospective patient.
Photo credit: Inspire Medical