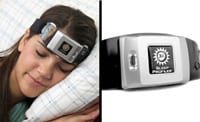
The headset, which employs neurostimulation, will undergo studies to evaluate its effectiveness in reducing insomnia and anxiety symptoms.
Summary: Nexalin Technology Inc announced that the FDA has agreed on the design for its planned clinical studies of the Gen-3 HALO Clarity, a non-invasive, deep intracranial frequency stimulation headset device, targeting insomnia and anxiety. Nexalin plans to commence pilot and pivotal studies in the third quarter, with each study involving 75 patients receiving active treatment and 75 receiving sham treatment. These studies aim to assess HALO’s effectiveness in reducing symptoms of insomnia and anxiety and build on prior positive clinical results. Upon completion, Nexalin intends to submit a De Novo request to the FDA for marketing approval.
Key Takeaways:
- The FDA has provided feedback and reached a consensus with Nexalin Technology on the design of clinical studies for the Gen-3 HALO Clarity device, which targets insomnia and anxiety.
- The initial pilot and pivotal studies are set to begin in the third quarter, each involving 150 patients to evaluate the HALO’s effectiveness in reducing insomnia and anxiety symptoms.
- Following the studies, Nexalin plans to submit a De Novo request to the FDA, aiming for a new marketing pathway to classify the HALO as a novel medical device, building on previous positive clinical results.
Nexalin Technology Inc announced that the US Food and Drug Administration (FDA) has provided feedback and reached a consensus on the design for its planned clinical studies in insomnia and anxiety for its new Gen-3 HALO Clarity, a non-invasive, deep intracranial frequency stimulation (DIFS) headset device.
After the studies are completed and evaluated, Nexalin plans to submit a De Novo request application for the HALO to the FDA. The De Novo request provides a marketing pathway to classify new novel medical devices.
Clinical Studies Set to Begin
The initial pilot and pivotal studies insomnia and anxiety are expected to commence in the third quarter. Each of the pivotal studies will include 75 patients receiving active treatment and 75 patients receiving sham treatment. They are intended to evaluate HALO’s ability to reduce symptoms of insomnia and anxiety, respectively.
These two studies are intended to build on the positive results of prior published clinical studies evaluating Nexalin’s Gen-3 HALO utilizing the new advanced DIFS waveform.
“We are proud to have arrived at a consensus with the FDA for the clinical protocols to evaluate the safety and efficacy of HALO in both anxiety and insomnia,” says David Owens, MD, chief medical officer of Nexalin, in a release. “This marks a major milestone for Nexalin and a significant step forward in the regulatory pathway. These studies will build upon our extensive prior published clinical studies in the US and Asia, which have repeatedly demonstrated the efficacy of our proprietary waveform.”
Mark White, CEO of Nexalin, adds in a release, “We have commenced a large production run of over 500 units, a key step that will support the FDA protocols for the planned studies. Overall, we believe the progress we have made is a major feat in a relatively short amount of time-from vision to design, pre-manufacturing to full manufacturing, and usability testing to protocol approval. We look forward to providing further updates on our clinical studies as developments unfold.”
Photo 140998720 © Nuttachai Wantanaboon | Dreamstime.com