 |
Technological advancement is perhaps the major factor enabling the move toward home sleep testing (HST). Such advances have made sophisticated monitoring quite feasible in the home for a subset of patients. Even when sleep staging with EEG, EOG, and EMG is not obtained, a plethora of other information is now available with some type III studies. With some of these more sophisticated type III devices, secure digital (SD) card recording and wireless transmission from the head box can totally eliminate the usual tethering and resultant sleep disruption. Portable monitoring can even include remote attendance with real-time viewing of all recorded channels and live videography as well. Communication to the patient by phone can facilitate correction of any displaced sensors. As evidenced in the following case studies, portable monitoring, when properly administered and carefully interpreted, can serve as an accurate testing modality in the resolution of sleep disorders.
CASE ONE
A 55-year-old man weighing 309 pounds at 5’10” presented with disruptive snoring, witnessed apnea, and marked sleepiness. He underwent a polysomnogram, which showed severe obstructive sleep apnea (OSA) controlled on 7 cm of CPAP. He used the device faithfully and embarked upon a weight reduction diet and exercise program. His weight decreased to 176 pounds, and he abandoned his CPAP therapy since he felt he no longer needed it.
However, his weight gradually increased to 236 pounds, and he was noted to have high blood pressure (HPT), diabetes, and hypercholesterolemia, requiring a number of medications, including injectable insulin. He also experienced exertional chest pressure. An urgent coronary arteriogram demonstrated severe coronary disease, but without evidence of myocardial dysfunction. He underwent and recovered uneventfully from his coronary artery bypass graft.
After discharge, because of postoperative nocturnal desaturations and his various comorbidities, his physician recommended evaluation for OSA. The patient refused an in-lab study, having had “enough of hospitals,” but did consent to a home test. He underwent type III monitoring in his home using CleveMed’s SleepScout™ (SS) illustrated in Figure 1. Monitoring results showed severe OSA with marked desaturation into the 70% range and apnea-related, multiple premature ventricular contractions (PVCs).
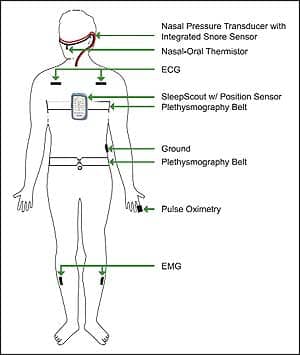 |
| Figure 1. Hook-up diagram for the CleveMed SleepScout, 9-channel type III portable monitor used in cases 1 and 2. CleveMed Crystal was used in case 3 and also provided EEG, EOG, and chin EMG. |
On the second night, he was monitored in his home utilizing Auto-PAP set in the automatic mode at a range of 5 cm to 15 cm, in tandem with the same SS monitor. Downloaded data from the Auto-PAP recommended a pressure of 7 cm. However, the portable monitor indicated that although his overt apneas had been corrected, there were still five hypopneas per hour with desaturations to 80% along with PVCs.
The third night the study was repeated, but at an increased and fixed pressure of 10 cm, and again with the portable monitor in tandem. This third night study demonstrated complete control of his hypopneas, desaturation, and arrhythmias.
This patient underwent a diagnostic study, home titration, HST validation, and CPAP education and dispensing, without ever leaving his home. The cost was a small fraction of the traditional in-lab approach, and it took place in 72 hours rather than several weeks. After several weeks on CPAP, he was weaned from insulin, and his HPT was readily controlled on a single drug. His disruptive snoring resolved, as did his sleepiness, and his Epworth Sleepiness Scale (ESS) normalized.
DISCUSSION
This case demonstrates great value in a home study. The patient had a dire need for a sleep study, but refused it. An HST made a study possible, and may have even saved his life. Despite the absence of sleep staging on this type III study, results showed his apnea was quite severe and dangerous. Had the second night of study been performed on Auto-PAP alone, long-term fixed CPAP of 7 cm would have been prescribed. Having additional data allowed identification of more subtle episodes—and could also have identified central and complex apnea—markedly enhancing the second night of study. It also allowed extrapolation toward an effective pressure. The advantage of studying on a third night allowed objective validation that 10 cm was ideal. His apnea was totally controlled, and his autonomic instability was alleviated as evidenced by his stabilization of heart rate variability and correction of desaturation and ventricular arrhythmias.
CASE TWO
The patient was the 55-year-old wife of case number one. She was a moderately obese (5′ 1″, 220 pounds) practicing nurse who was noted to snore and would awaken at night choking and gasping. She would assume the prone position in an effort to improve her sleep quality. Her sleep was not entirely refreshing, although her daytime sleepiness was only mild.
On night one, this patient also underwent a baseline study utilizing the SS, recording the parameters illustrated in Figure 1. She fell just short of a firm sleep apnea diagnosis, because the respiratory distress index (RDI) was only 4 per hour of recording time (not sleep time). Because her baseline saturation was 98%, her hypopneas did not meet the CMS requirement of 4% desaturation. The respiratory episodes that were scorable occurred in clusters an hour and a half apart, suggesting they were REM-related hypopneas.
On night two, Auto-PAP titration was performed similarly to that for her husband, in tandem with the SS, but she had a very different experience. Since this was her first time on CPAP and she was a stomach sleeper, she had a difficult time getting comfortable with her nasal mask. Presenting even greater difficulty was the volume of air that was continuously being delivered to her. Although the device was set in the auto-titration mode during the entire recording period, it titrated itself to a fixed pressure of 13 cm due to mouth leak. Because of the high pressure, the patient did not sleep at all, yet the Auto-PAP misconstrued that sleep hypopneas were occurring in spite of the pressure and her awake state. The graphic results are shown in Figure 2.
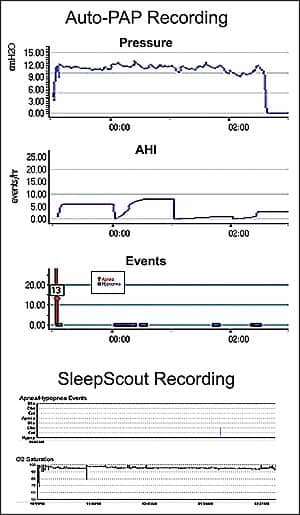 |
| Figure 2. The upper panel Auto-PAP recording mistakenly shows frequent hypopneas and an excessive pressure requirement of 13 cm. The bottom panel from the in-tandem type III device shows no hypopneas actually occurred. |
On the third and last night, the Auto-PAP was decreased to a range of 4 cm to 7 cm, and the patient actually slept quite well for what she estimated to be an 8-hour period. The portable monitor parameters suggested restful sleep without hypopneas. She woke the next morning feeling much more refreshed than usual, and continued to improve on maintenance CPAP. In order to qualify for CPAP reimbursement, she required an in-lab study. It indexed the hypopneas for true sleep time and allowed scoring of the non-desaturating hypopneas because of the resultant arousals. Her in-lab AHI nearly quadrupled to 15, rather than what was suggested by the HST’s RDI of 4, making the diagnosis of moderate OSA and justifying CPAP coverage. She is now much improved in all regards on her maintenance CPAP of 7 cm. Until her OSA was corrected, she states she didn’t realize how sleepy she really was.
DISCUSSION
This case illustrates a potential pitfall of a type III HST. Due to dilution by wake time and the absence of recordable arousals to identify hypopneas, the RDI was a fraction of the AHI identifiable by the in-lab PSG. Clinical suspicion prevented the screening HST from being a diagnostic end-point, and led to the appropriate in-lab PSG.
On night two, conclusions from the Auto-PAP were worse than a failure. It concluded that there was a need for a high pressure, and even then, it incompletely treated her hypopneas. In fact, the high pressures titrated by the Auto-PAP were due to both her wake state and the massive mouth leak. This was revealed by the add-on type III SS device, which refuted the presence of real hypopneas. Auto-PAP titration alone, without the portable monitor, would have resulted in misdiagnosis and inappropriate therapy. This patient’s case illustrates that home titration is not for everyone, although her third night on a lower pressure did indicate that she could benefit from low-dose CPAP.
CASE THREE
A 78-year-old man was a resident of a nursing home for a variety of medical problems. He also was reported to have mild snoring and minimal daytime sleepiness and stated that he spent almost all night, every night, completely awake. He underwent a complete PSG including not only the parameters in Figure 1, but also utilizing CleveMed’s Sapphire PSG™. It included full EEG, EOG, and EMG, along with real-time data and video monitoring from a remote location. The patient slept for 300 of the 400 minutes of recording time, giving him a sleep efficiency of 75%. His sleep latency was 16 minutes. His wake time after sleep onset was 58 minutes, which was due to two prolonged awakenings for bathroom trips. He spent 14% of his sleep time in slow wave sleep and 12% in REM (Figure 3).
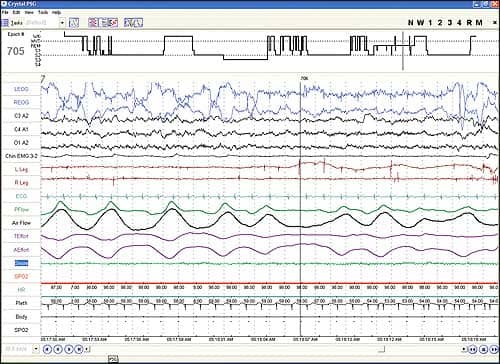 |
| Figure 3. Screenshot of Crystal epoch shows REM sleep, although the patient felt he was awake all night, indicative of paradoxical insomnia. |
On his morning questionnaire, he stated he had a typical sleepless night. He estimated his sleep period was less than 1 hour total. When he talked to the attending technologist on bathroom breaks during the study, he stated that he had not slept at all, despite good sleep recorded on the PSG. With a total recorded sleep time of 5 hours, this was pathognomonic of sleep state misperception or paradoxical insomnia. This study demonstrates an excellent example of how a nursing home patient requiring a full sleep study could be monitored with sleep stage parameters and attendance by a technologist. All of this was accomplished remotely and the cause of his perceived insomnia was made clear.
CONCLUSION
Prudent application of home testing can potentially bring the undiagnosed population with sleep problems under mainstream medical care. Rather than being the end of quality sleep care as we know it, it can actually enhance it. It can be used to identify those who not only suffer from the symptoms of sleep disorders, but are at risk for resultant poor health outcomes, and are therefore major consumers of health care resources. HST can be used to validate the adequacy or inadequacy of therapy in both the short and long term. It can be used to provide continuity of care as an objective tool of benefit or lack of it. Judicious application can accomplish these goals while saving an enormous amount in health care dollars. This is a win-win situation, providing more care to more patients, for less cost, yet at a high level of quality.
Technology and innovation have made all of this possible. However, HST puts a much greater onus on the human ingredient in this equation. Clinical evaluation must be stringent enough to identify who is and who is not a candidate for home study. Careful scrutiny of data, which on the surface may seem to be quite simple, will be required by skilled interpreters to identify which studies indeed are either inadequate or inaccurate. Strength of conviction will be necessary to push forward to the more expensive and seemingly redundant in-lab studies in those who need them, despite patient or payor resistance. Perseverance in providing long-term continuity of care and follow-up, both clinically and objectively using HST, will be essential to sustain benefit. Payors must be willing to pay the tariff for the necessary level of study, and repeat studies when required.
While HST is not for everyone, it can be a valuable tool that needs to be individually tailored to a given patient at a particular time in the course of their evaluation, treatment, and follow-up. Greater skill, discretion, and clinical judgment will be required by technologists and sleep specialists alike in order to allow this technology to meet its promise.
Joseph Golish, MD, is a board-certified specialist in sleep medicine and author of more than 300 publications on sleep. He is a member of the Sleep Steering Committee for the ACCP. After 35 years as a professor at Cleveland Clinic and head of sleep medicine, he has left academic medicine to advance a new paradigm in sleep medicine, focusing on accessibility and affordability. His goals are the proper use of HST and fostering continuity of care, in an efficient and cost-effective manner, while preserving high quality. He is currently the medical director of both Cleveland Medical Devices (CleveMed) and Circadia Sleep Center in Cleveland. He can be reached at [email protected].




