The evidence that OSA treatment may help prevent cardiovascular disease increases.
| No Simple Cure By Lena Kauffman |

When grouped together, the cardiovascular diseases are the leading cause of death, disability, and health care expenditure in the United States and the leading cause of mortality worldwide.1 It is estimated that one quarter of Americans live with CVD, and the burden of disease and economic impact is projected to grow as the population ages and risk factors, such as obesity and diabetes, continue to rise.2 Other major modifiable risk factors include high blood pressure, abnormal blood lipids, smoking, diabetes, and unhealthy diets. Nonmodifiable risk factors include advancing age, family history, and male gender.
OSA shares many of the same risk factors as CVD such as male gender, obesity, and hypertension; however, OSA does occur in both sexes, thin people, and all age groups including children. In 1992, OSA was estimated to occur in 24% of men and 9% of women aged 30 to 60 years and OSA syndrome was found in 4% of men and 2% of women.3 Given the increase in the prevalence of obesity since 1992, it is likely that these numbers also have increased.
The Sleep Heart Health Study, comprising the largest cohort of community dwelling adults (n>6,000) studied longitudinally for the presence of sleep-disordered breathing and its consequences, found that participants with mild to moderate sleep apnea had elevated cardiovascular risk factors at the time of the baseline sleep study. Across all age groups and genders, participants with higher respiratory disturbance indexes (RDIs) had higher body mass indexes, waist-to-hip ratios, and prevalences of hypertension and diabetes. In participants less than 65 years old, the increase in RDI was also associated with increased levels of triglycerides and lower levels of high-density lipoprotein (HDL) cholesterol. Much of the increase in CVD risk, except hypertension, was attributed to the presence of obesity.4
OSA and Hypertension
Despite the complex relationship between OSA and CVD, the interaction of coexisting risk factors and pathophysiologic mechanisms, scientific evidence linking OSA to the development of systemic hypertension is compelling. Increased sympathetic nervous system activity, reduced baroreceptor sensitivity, and endothelial dysfunction have all been associated with OSA and are also implicated in the pathogenesis of hypertension. Animal studies have demonstrated that experimentally induced OSA can cause sustained hypertension5 while human epidemiological studies have confirmed that untreated OSA increases the risk of having hypertension6 or, if normotensive, developing hypertension even when controlling for confounding variables. Prospective data from the Wisconsin Sleep Cohort Study reported over a 4-year follow-up period an almost threefold increase in the development of hypertension for participants with an apnea plus hypopnea index (AHI) of more than 15 events per hour at baseline.7
Application of CPAP stabilizes the upper airway, preventing collapse and the acute cardiovascular and hemodynamic consequences of OSA. Repetitive surges in blood pressure associated with the termination of obstructive events are attenuated, and well-controlled randomized studies of CPAP applied over several weeks report that it reduced both daytime and nocturnal mean systolic and diastolic blood pressure.8 Studies also report reductions in mean systolic and diastolic blood pressure in the order of approximately 10 mm Hg. These reductions are predicted to reduce stroke risk by 56% and coronary heart disease event risk by 37%.9
The United States National Heart, Lung, and Blood Institute now recognizes OSA as a significant and reversible cause of hypertension. Given that studies report a high prevalence of OSA in patients with uncontrolled hypertension and that considerable overlap exists in the risk factors for these conditions, careful evaluation of the patient history and clinical data for the presence of sleep apnea is warranted in all overweight hypertensive patients.10
OSA and Ischemic Heart Disease
Recent studies confirm that untreated OSA increases the risk of cardiovascular events in patients with existing ischemic heart disease (IHD) independent of other confounders.11,12 In one prospective cohort study of 408 participants, with a median follow-up of 5.1 years, patients with an AHI greater than 10 had a 62% relative increase and a 10.1% absolute increase in the risk of cardiovascular events (myocardial infarction, cerebrovascular events, and death).11 Similarly, the Sleep Heart Health Study reported that sleep apnea was associated with an increased risk of several CVD outcomes, including myocardial infarction and stroke.6
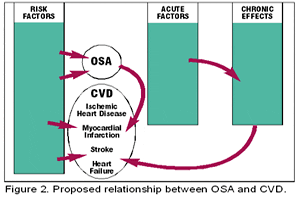
The acute effects of OSA can be seen in Figure 1 on page 34, which shows intermittent hypoxia and repetitive sympathetic activation associated with cyclic fluctuations in heart rate and blood pressure. The combination of increased myocardial oxygen demand together with apnea-induced myocardial ischemia may contribute to the increased risk of sudden death from cardiac causes during sleeping hours in patients with OSA.13 Large negative intrathoracic pressure swings combined with surges in blood pressure are associated with large increases in myocardial transmural pressures promoting left ventricular hypertrophy, a known predictor of CVD events even in the absence of daytime hypertension.14
Although the pathophysiologic mechanisms linking OSA to IHD are unclear, there is evidence to suggest that hypoxia may be important in triggering a generalized inflammatory response. Endothelial damage and dysfunction associated with the inflammatory response are important in the pathogenesis of atherosclerosis, probably via increased production of reactive oxygen free radicals, reduced endothelial derived nitric oxide production, and impaired vasodilation.15-17
Congestive Heart Failure
Congestive heart failure (CHF) is a potential end point of a variety of serious heart diseases most commonly resulting from progressive impairments in contractile function (systolic dysfunction) often caused by ischemic injury. Excessive pressure-volume loads imposed on the heart from conditions such as hypertensive heart disease are a major cause of diastolic dysfunction—a state in which abnormalities in ventricular relaxation and stiffness occur.
CHF is associated with poor prognosis with patients often complaining of paroxysmal nocturnal dyspnea, orthopnea, anxiety and depression, fatigue, and poor sleep. The medical community now recognizes that there is a high presence of sleep-disordered breathing in this patient group with some studies reporting a prevalence of 50% to 70% depending on AHI and the degree of cardiac impairment.18,19 In addition, researchers have found that approximately 35% of diastolic20 and 30% of systolic21 CHF patients have OSA—a prevalence much greater than that observed in the general community.
The Sleep Heart Health Study reported that patients in the upper quartile of OSA severity had the highest adjusted relative risk for self-reported heart failure. Community dwellers with an AHI greater than 11 events per hour had a relative risk of 2.4 for reporting a history of CHF compared to those with an AHI of less than 1.4.6 In a further study of 169 randomly selected patients with established OSA, about 8% demonstrated a left ventricular ejection fraction (LVEF) of less than 50%22 while diastolic dysfunction was reported in 37% of 68 patients with OSA.23 Although these prevalences vary widely, conservatively more than 10% of patients with OSA will have some type of systolic or diastolic left ventricular (LV) impairment.
The mechanisms linking OSA to CHF are poorly understood; however, marked negative intrathoracic pressure swings and hypoxia produce adverse acute hemodynamic and neurohumoral effects, and when repeated over months to years, susceptible individuals may develop sustained LV dysfunction.
Canine studies show that a single night of intermittent upper airway occlusion results in electron microscopic evidence of pulmonary edema,24 and, when the repetitive apneas continue for 1 to 3 months, impaired LV systolic function and hypertension develop.25 In humans with OSA but without CHF, the alveolar-arterial Po2 gradient widens from the beginning to the end of sleep,26 suggesting that subtle pulmonary edema develops in these people.
Central Sleep Apnea
The majority, 30% to 55% of symptomatic and asymptomatic CHF, patients suffer from central sleep apnea (CSA) or Cheyne-Stokes respiration (CSR). Instead of upper airway collapse, a waxing and waning pattern of ventilation results from fluctuations in respiratory drive resulting from increased circulatory time and increased sensitivity of the central and peripheral chemoreceptors.28 CSA is considered a consequence of heart failure itself and, when present, is associated with a poor prognosis, attributed to similar pathophysiologic influences that occur in OSA. Intermittent hypoxia, elevated sympathetic activity during both wakefulness and sleep,29 and increased pulmonary capillary wedge pressure (PCWP) are observed,19 and despite reductions or absence of intrathoracic pressure swings during the apneic or hypopneic phase, myocardial transmural pressures are also increased.
At present, there are no epidemiological studies regarding the prevalence of CSA in the general population; however, it is also reported in normal subjects at high altitude and in patients with long-term opioid use, and rarely an idiopathic variety is observed. When CSA with a long cycle length of approximately 1 minute occurs, or the OSA pattern changes to CSA in patients with no history of cardiac failure, one should strongly suspect asymptomatic LV dysfunction.
Sleep Apnea Treatment’s Effect
Treatment of OSA and CSA should be considered important in the management of patients with heart failure as the hemodynamic and neurohumoral consequences of untreated sleep apnea may contribute to cardiac decline.
Randomized controlled trials of CPAP in patients with CHF and OSA have shown improvements in LVEF; reductions in blood pressure, LV chamber size, and catecholamine levels; as well as improvements in quality of life following CPAP use.30,31 Partial reversal of LV diastolic dysfunction has also been reported following 12 weeks of CPAP treatment in a group of patients with newly diagnosed OSA and otherwise free of existing CVD.32
Similarly, in patients with CSA, improvements have been observed in cardiac output, symptoms of dyspnea and fatigue, inspiratory muscle strength,33 circulating catecholamines,34 as well as survival.35
Whether weight loss, upper airway surgery, mandibular advancement splints, or more sophisticated forms of nocturnal pressure ventilation have a role to play with cardiac function in CHF needs further exploration.
Conclusion
The evidence for OSA as an independent risk factor for the development of CVD is now convincing with increasing evidence emerging from intervention studies that examine the possible pathophysiologic links between these conditions and add further support to the epidemiologic evidence available to date. Responsible mechanisms include hypoxemia, negative intrathoracic pressures, autonomic imbalance, and vascular endothelial dysfunction. Such patients are likely to benefit from the relief of OSA by CPAP. In patients with established CHF, CSA is a marker of advanced disease, which may also be responsive to positive airway pressure.
| No Simple Cure
by Lena Kauffman As the links between sleep apnea and heart disease become more apparent, the importance of treating sleep disordered breathing is increasing. However, treating sleep apneas in patients who already have heart failure is no easy task. Traditional CPAP in patients with advanced heart failure creates two issues, says Lee R. Goldberg, MD, assistant professor of medicine in the cardiology department of the University of Pennsylvania, Philadelphia. The first is comfort. Because heart failure makes these patients short of breath, a mask can feel smothering and even a slowly increasing positive airway pressure can be uncomfortable. The second is a theoretical concern that the CPAP may do more harm than good in patients who have periodic breathing patterns, such as Cheyne-Stokes respiration, where patients cycle between periods of apnea and periods of hyperventilation. “When they are not breathing very well the CPAP is partially effective in helping to correct that, but Cheyne-Stokes respiration has two phases,” Goldberg says. “One is a phase where they are breathing much too quickly and one is a phase when they are not breathing enough, and during the phase when they are breathing much too quickly, the CPAP may actually make that worse by supporting respiration and actually augmenting the part where we don’t want them to breathe as much.” The problem with hyperventilation in patients with heart disease is that it could put them at risk of other complications, such as arrhythmias and electrolyte shifts, Goldberg says. Fortunately, advances in cardiac care have decreased the number of patients with advanced heart disease and central sleep apnea with Cheyne-Stokes respiration, says Peter Gay, MD, director of the pulmonary laboratory at the Mayo Clinic in Rochester, Minn. Whereas earlier smaller studies found the number of patients with significant congestive heart failure that had Cheyne-Stokes respiration to be as high as 50%, Gay thinks central sleep apnea probably represents about 10% of the overall population of sleep disordered breathing and those with Cheyne-Stokes respiration are a small minority within that 10%. For that group, however, treatment options have been limited. “Even at Penn where we do a lot of sleep [medicine and there is] a lot of cooperation between cardiology and sleep, I don’t think we had a consensus of how to treat them,” Goldberg says. Often patients were simply put on nocturnal oxygen. “There was an acknowledgement that it was probably not the ideal therapy, but, short of having anything else available, that was what most of the patients had,” he says. Another option was using a bi-level device with a timed backup rate, Gay says. However, even in patients where this device did help, he found that some still had residual breathing problems. Fortunately, technological advances made in positive airway pressure technology may soon create additional options for these patients. Goldberg and Gay both recently finished separate studies involving an adaptive servo ventilator that automatically adjusts pressure levels in response to waxing and waning breathing patterns, such as those seen in Cheyne-Stokes respiration. Like all positive airway pressure devices, the device, which was recently cleared by the Food and Drug Administration, may not work for all patients and a special concern in heart-failure patients is extremely low blood pressure, which in some patients drops even further when they are put on a positive airway pressure device, Goldberg says. However, for about a third of the patients in his clinical trial of the device, it was so effective that the patients were reluctant to give it up at the end of the trial. “We had others who struggled with it and we had to do a lot of coaching, so it certainly wasn’t everybody, but there were patients who really felt like ‘wow,’ this has made a big difference for them,” he says. At the University of Colorado Health Sciences Center in Denver, assistant professor of cardiology Simon Shakar, MD, also had two of the 13 patients he tested on the device feel better and one who felt so much better that Shakar petitioned to allow him to keep the device after the study. However, Shakar was cautious about interpreting too much from this anecdotal evidence. When it comes to patients with Cheyne-Stokes respiration, more work needs to be done in a greater population of patients to tease out the specific benefit of the device from the other therapies (both pharmacological and respiratory) that some of the patients in the current studies were receiving for their heart disease, he says. However, even if research does not eventually show all of the benefits of the device that the developers may hope for, Shakar says that more sophisticated positive airway pressure devices are a step forward in making patients with all types of sleep apneas more comfortable. Currently, Shakar found getting his patients compliant with existing devices to be a challenge. “A lot of them hate CPAP,” he says. Gay is also already thinking of the potential for this new device to help patients with other types of apneas that are currently difficult to treat. “With the study, we have since become aware that there is a notable group of patients that may have a component of obstructive sleep apnea, but they have a disorder of the response to positive airway pressure similar to the Cheyne-Stokes patients,” he says. “When you give them conventional CPAP, they actually develop a marked increase in the number of central apnea events and these patients responded very favorably to the new device.” |
Matthew T. Naughton, MD, FRACP, is the head of general respiratory and sleep medicine at the Alfred Hospital, Melbourne, Australia, and associate professor in the Department of Medicine, Monash University; Irene Szollosi, is a Monash University PhD student at the hospital, and is supported by an Australian Postgraduate Award.
References
1. Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases, Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855-2864.
2. Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the National Conference on Cardiovascular Disease Prevention. Circulation. 2000;102:3137-3147.
3. Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230-1235.
4. Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: The Sleep Heart Health Study. Am J Epidemiol. 2001;154:50-59.
5. Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106-109.
6. Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19-25.
7. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
8. Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomized parallel trial. Lancet. 2002;359:204-210.
9. Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68-73.
10. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
11. Mooe T, Franklin K, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease. Long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910-1913.
12. Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index. An independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81-86.
13. Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206-1214.
14. Leung R, Bradley D. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147-2165.
15. Ip MSM, Tse HF, Lam B, Tsang KWT, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348-353.
16. Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607-2610.
17. Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123-128.
18. Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154-2159.
19. Solin P, Bergin P, Richardson M, et al. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574-1579.
20. Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111:1488-1493.
21. Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101-1106.
22. Laaban JP, Pascal-Sebaoun S, Bloch E, et al. Left ventricular systolic dysfunction in patients with obstructive sleep apnea syndrome. Chest. 2002;122:1133-1138.
23. Fung JWH, Li TST, Choy DKL, et al. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest. 2002;121:422-429.
24. Fletcher EC, Proctor M, Yu J, et al. Pulmonary edema develops after recurrent obstructive apneas. Am J Respir Crit Care Med. 1999;160:1688-1696.
25. Parker JD, Brooks D, Kozar LF, et al. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. 1999;160:1888-1896.
26. Guardiola J, Yu J, Hasan N, Fletcher E. Evening and morning blood gases in patients with obstructive sleep apnea. Sleep Med. 2004;5:489-493.
27. Cloward TV, Walker JM, Farney RJ, Anderson JL. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with nasal continuous positive airway pressure. Chest. 2003;124:594-601.
28. Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167-182.
29. Solin P, Kaye DM, Little PJ, et al. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest. 2003;123:1119-1126.
30. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241.
31. Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361-366.
32. Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375-383.
33. Granton J, Naughton M, Benard D, et al. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996;153:277-282.
34. Naughton M, Benard D, Liu P, et al. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473-479.
35. Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61-66.




