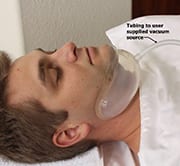
Sommetrics, a late-stage development company focused on bringing a new treatment for sleep apnea to market, presented updates on the progress of its flagship product, the aerSleep II system, aimed at treating moderate and severe sleep apnea, during the 22nd Annual Needham Virtual Healthcare Conference.
aerSleep II, a cordless neck collar, works by opening the airway from the outside with a gentle negative-pressure vacuum. The portable device features an integrated, silent vacuum pump and is held in place without the need for a retaining strap.
Sommetrics CEO Richard Rose says in a presentation during the healthcare conference that the company currently is closing a series D financing round of $20 million, which it hopes to close by mid-year, and proceeds will be used to finish the pivotal trial for aerSleep II, file for market clearance, and prepare to commercially launch in the US pending FDA clearance. The company is targeting a 2025 US launch.
Interim data from the company’s current pivotal SUPRA (Study Using Negative Pressure to Relieve Apnea) trial and eight earlier studies demonstrate that aerSleep II is effective and well-tolerated. Data shows the technology is effective in over 70% of people with all levels of disease, with no major safety issues found. After three weeks of home use, 76% of patients preferred aerSleep to their current or previous treatment, according to data from the company.
Launched in the fourth quarter of 2021, the SUPRA trial focuses on individuals with moderate to severe sleep apnea who struggle with CPAP. Enrollment is expected to be completed in 2024. Subjects are entered into a six-month home treatment phase if they acclimate to the device and exhibit a favorable treatment response (50% reduction in apnea-hypopnea index from baseline and absolute apnea-hypopnea index <20/hour) during initial home sleep testing. The primary endpoint is to demonstrate continued treatment efficacy at six months of home therapy.
According to Rose, data shows a clinically meaningful reduction in sleep apnea-related impairment, continued benefit after six months of home use, and all subjects have exceeded Medicare compliance criteria.