Inspire Medical Systems Inc this month announced the results of a landmark long-term clinical study for its Inspire Upper Airway Stimulation (UAS) System, the first FDA-approved implantable neurostimulation treatment for people diagnosed with obstructive sleep apnea (OSA).
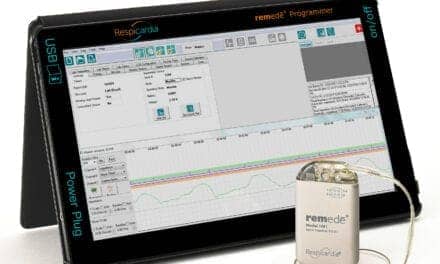
Inspire therapy is for some people diagnosed with moderate to severe OSA who are unable to tolerate or get relief from CPAP. In contrast to CPAP, Inspire therapy works inside the body and with a patient’s natural breathing process. Controlled by the patient sleep remote, the system includes a breathing sensor and a stimulation lead powered by a small battery. During sleep, the system senses breathing patterns and delivers mild stimulation to the tongue and other soft tissues of the throat to keep the airway open. Inspire therapy is currently available at more than 60 medical centers across the United States and Europe.
The Stimulation Therapy for Apnea Reduction (STAR) trial was conducted at 22 sleep medicine centers across the United States and Europe. One-year STAR trial outcome measures, published in the January 9, 2014 edition of the New England Journal of Medicine, showed that sleep apnea patients receiving Inspire therapy experienced significant reductions in sleep apnea events and significant improvements in quality of life measures.
The new long-term study outcomes showed that the improvements observed at one-year were sustained at the three-year follow up mark. The outcomes include:
- A 78% reduction in apnea-hypopnea index (AHI) from baseline
- An 80% reduction in oxygen desaturation events from baseline
- 80% of bed partners reported soft or no snoring as compared to 17% of bed partners at baseline
- Quality of life measures, including daytime sleepiness and functioning, showed clinically meaningful improvements and a return to normal levels over baseline
The results were published in the online issue of Otolaryngology – Head and Neck Surgery, the official peer-reviewed publication of the American Academy of Otolaryngology – Head and Neck Surgery Foundation.
New data from the STAR Trial demonstrate that more than 80% of the patients with Inspire therapy report nightly use after 3 years of being prescribed the therapy.
The data was recently presented at the annual American Academy of Otolaryngology – Head and Neck Surgery Foundation meeting in Dallas by B. Tucker Woodson, MD, chief of the Division of Sleep Medicine at Froedtert Hospital and the Medical College of Wisconsin. “The data confirms that Inspire Upper Airway Stimulation therapy is safe and effective and that the results are consistent over the long term,” says Woodson, lead author of the 36-month manuscript, in a release. “We also observed high therapy adherence rates throughout the three-year STAR trial follow-up period. It is exciting to have an effective treatment to help those sleep apnea patients who are not able to tolerate or achieve consistent benefit from CPAP.”
Tim Herbert, Inspire Medical Systems CEO, says, “The three-year study outcomes are significant as they demonstrate that improvements in both objective respiratory and subjective quality of life measures are maintained. We thank and congratulate both the STAR trial investigator group and the author team for completing the long-term follow up and publishing these important outcomes.”




I would like a booklet or liturer on this subjet if I could.
Please send me more info in this device.
I need more information.
My ENT prescribed this devise for my severe sleep apnea. I am an excellent candidate but my insurance won’t cover the cost due to it being experimental. Is there a time frame as to when it won’t be considered experimental?
It’s no longer experimental. Visit Inspire’s website or contact your ENT/Sleep Doctor. I’m looking into it for my dad and his Medicare advantage plan will cover it (Blue Cross, Blue Shield). Good luck!
I wish my insurance company would cover the procedure. I do not have $50,000 to spend on this.
What is the price range for this procedure?
How does the price of CPAP compar
Is there a list of which providers cover this procedure?
I would like.some information about this new treatment inspire.is it done here in ireland or where in Europe and how much does it cost to have this procedure.thank you
What is the cost for this procedure. i currently have cpac very uncomfortable and noisy. Let me know.
insurance coverage?
PLEASE EMAIL THE ARTICLE DESCRIBED ABOVE AS WELL AS ANY OTHER PERTINENT INFO YOU MAY HAVE AVAILABLE. THANK YOU!
I will be meeting with my VA doctor next week to discuss Inspire Sleep. I need to know more about the implant process. I am currently wearing an implanted pacemaker.
Jack
Please feel free to call me at 248-231-2897
I’m a Vietnam vintage Marine that has severe
Seep Apnea It’s my understanding the VA will pay for this procedure. I’d love to hear your findings and what you’ve gone through.
Kind Regards,
I have been fighting with Anthem BC of Virginia for over 3 years to get this procedure done. They call it Experimental and won’t pay for it. I now have a different job in VA with another form of Anthem. My doctor filed the claim to see if they would cover and it was denied. I filed an appeal. I will find out on Friday if that is denied. Then I will check to see if I have the right to file an Outside Appeal. If not, I am out of luck. Can’t afford it without Insurance. Fighting with Anthem is mind numbing! How can an FDA approved device that has been around for years and been implanted in thousands of patients be experimental? I’ll give up if it doesn’t pass this time. Anthem told me that they were Grandfathered into the ACA last time. Nice.
IT SHOULD NOT BE A MYSTERY THAT YOUR INSURANCE CARRIER DOESN’T WANT TO PAY $30,000-$40,000 PLUS AN ADDITIONAL $17,000 FOR INSTALLATION. MY ADVICE IS TO DO SOME RESEARCH ON INSURANCE COMPANIES THAT WILL FUND THIS PROCEDURE,PREFERABLY STATE RUN MEDICARE AND MEDICAID PROVIDERS. YOUR INSURANCE COMPANIES SOUNDS AS THOUGH ITS A SMALL COMPANY THAT PROBABLY DON’T PROVIDE MEDICAID OR MEDICARE. IF THERE IS A WILL THERE IS A WAY.HAVE BRUTE DETERMINATION. GOOD LUCK
I had the same experience with BCBS, but the medical company- Inspire- assigned me an advocate who was excellent walking me through the appeal process. Insurance company’s decision was eventually overturned after an outside appeal. From start to finish, it took 6 months. Two weeks after approval, I had the surgery, so even though it is maddening, hang in there with the process, hopefully you will be assigned an advocate.
For those of you that have had this treatment, have you found it to be successful? I am in the beginning stages to see if I can qualify. I am just curious if you feel its a success?
I would like more information on this procedure.
What is the process
Would like to learn more about the dangers and if it can be removed if you need an MRI.
thank you.
Would like to hear reviews from actual users. Does it make a difference for you? Do you feel like you slept? Have you tracked your sleep? What about getting a MRI? Does it set off airport scanners? What are the pros and cons? Do you feel it under your skin? Has it damaged your tongue and or throat from delivering the shocks? Does your body accept it or treat it like an invader? Are there side effects? I can’t find real reviews by users, but there are plenty by the vendors. Would love to hear “The rest of the story”. Thank you
I had Inspire implanted on June 17 and this past Monday (after healing from the surgery) they turned it on. I have had a great outcome. Sleeping much better and my husband is back in our bed after years of very loud snoring. Yes a little discomfort in my throat but feels better and better every morning.
How are you doing with it today?
I am considering the inspire sleep apnea procedure and would like to talk to someone who has had it. 419-892-3320
I found this method to fight sleep apnea useless.
I think the device is not ready to be used on the general public. I have the implant for several years and it’s unusable.
Removing it is my only option.
CPAP is the only real treatment.
.Do you still have the inspire implant? I’m struggling terribly with sleep apnea, low oxygen & waking with cluster migraine every day. I’m on by pap now but my airway closes while I sleep. I don’t know what to do. I am a candidate for inspire surgery
If the Inspire Device doesn’t work, can it be removed?
I have a pacemaker. Do I qualify for Inspire?
After 30 months with Inspire…
This is what I read about Inspire before I had my own: “The Inspire therapy works inside your body with your natural breathing process to treat obstructive sleep apnea. It continuously monitors your breathing patterns while you sleep. Based on your unique breathing patterns, the system delivers mild stimulation to key airway muscles which keeps the airway open while you sleep.”
From that description, I assumed there would be stimulation when my breathing paused causing an apnea event. The way mine actually works is that there is an initial pause in stimulation to allow you to fall asleep then the initial start-up can be a real jolt. My mouth is propped open by my tongue thrusting forward followed by a constant thumping of my tongue against the roof of my mouth. Only one side of my tongue is fully stimulated which causes the tongue to thrust out and to the side. Each stimulation is instant causing the tongue to jerk very fast. It also causes my tongue to buzz somewhat like it was AC current or chopped DC.
My tongue thrusts out for 3 seconds and then repeats after a one-second pause causing the constant thumping noise against the roof of my mouth. However, after 30 minutes to one hour, the tongue movement seems to settle down to a less aggressive and less jarring thumping. This may be simply a tiring of my tongue muscles rather than a change in the stimulation. The stimulation cycle of three seconds on and one second off eventually changes but never seems to have any coordination with my breathing. Sometimes it becomes quite rapid, and sometimes it lengthens enough to allow a complete breath. However, 90% of the time it simply follows the 3-second pulse.
I also noticed that it will not work at all if my electric blanket is turned on. And, I noticed that sometimes my electric shaver will turn on the stimulation. Experiencing this, makes me want to carry the remote with me everywhere in case the system turns on. I have also found the Patient Manual on the internet (which I now wish I had seen before having the surgery) which states that stimulation can initiate if you are near a CB Radio, electric welder, other electrical devices, dental drills, or near our induction stovetop. This is not so bad for me because I have an excuse not to help my wife in the kitchen, but bad news for not being able to use the handheld 2way CB radios that I use hiking or the welder in my shop. I will also need to remember to take control with me when I go to the dentist.
Before having the Inspire device I used a mouthpiece that holds my lower jaw forward as well as a BIPAP unit set at a fairly low pressure. I have to use a low pressure because raising the pressure results in severe gas pains for me as air flows into my stomach. The plus with using the BIPAP even at low pressure, however, is that I have online access to the daily report of the previous night’s sleep. I average 17.5 apnea events per hour using these two together.
I found if I use the Inspire device without the mouthpiece or the BIPAP, I wake in the night with a headache. Using the BIPAP, the mouthpiece, and the Inspire device solves the headache problem but does not change the average number of events per hour. It does add two inconveniences. First, frequently when the Inspire initiates the stimulation I wake up. This is almost always the case if I have it set on one of the highest values. The second is that I have always experienced sinus drainage, especially when I lie down. When this drainage is in my throat, I swallow reflexively. Using the Inspire device, I cannot swallow. A third problem is that the sensor pokes painfully in my ribs if I try to sleep on my right side.
My summary is that the device has been a disappointment for me. Because I still use the BIPAP on low pressure and can access a daily report on events per hour, I have repeatedly experimented with using Inspire and not using Inspire to see if it helps. My repeated data shows events per hour are the same using the Inspire or not using Inspire. In other words, the Inspire does not help me. I am also surprised to see a device being marketed with such a susceptibility to EMC/EMI interference. UL approval of the device would have revealed these problems. There should be a ramp of the initial stimulation over a period of minutes to prevent the initial jolt of full stimulation that wakes me up. There should also be a ramp of each stimulation to allow a more gentle tongue movement. And, of course, the sensitivity to electronic interference needs proper filtering. And finally, there should be an on/off switch on the control to keep the battery from going bad while you are carrying it with you.
In summary, the Inspire unit is an extremely poor implementation of an intriguing concept. I would not recommend it to anyone.